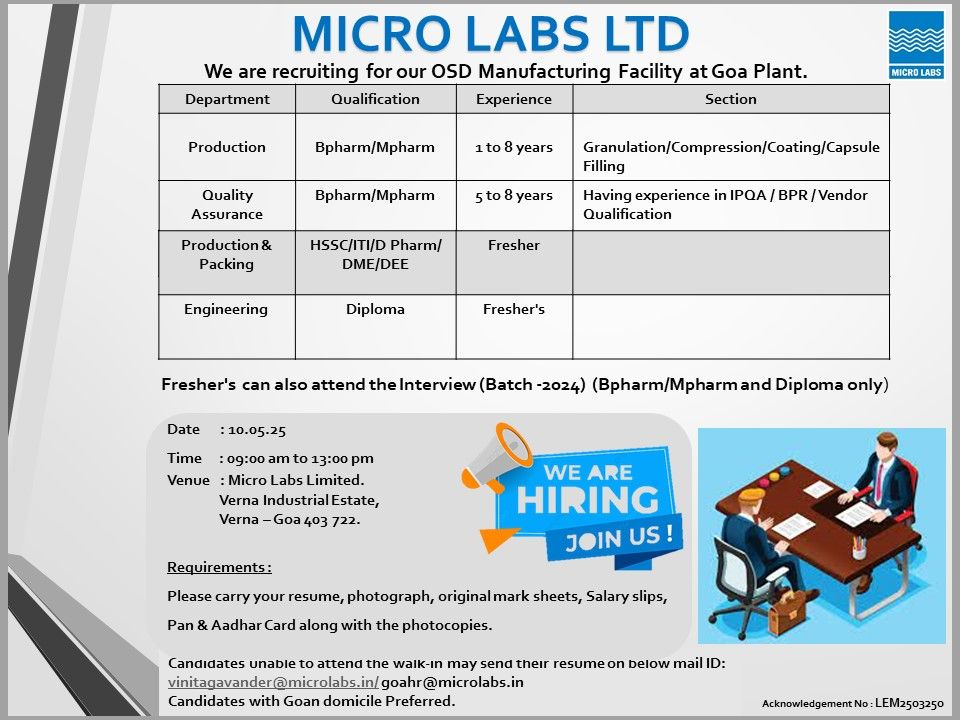
Join Micro Labs Limited, a USFDA-approved leader in oral solid dosage (OSD) formulations, at our Walk-In Interview on May 10, 2025, for Production, Quality Assurance (QA), Packing, and Engineering roles at our Verna, Goa facility.
Operating in 60+ countries with ₹3,500 Crore revenue (FY24), Micro Labs employs 10,000+ professionals and is rated 3.6/5 on AmbitionBox (2,200+ reviews) for job security. Salaries range from ₹2–8 Lakhs/year (Glassdoor). Be part of our mission to deliver affordable, innovative medicines
Walk-In Interview Details
- Date: Saturday, May 10, 2025
- Time: 9:00 AM – 1:00 PM
- Venue/Work Location: Micro Labs Limited, Plot No. S-155-S-159, Phase III, Verna Industrial Estate, Verna, Goa 403722
- Application: Walk-in with documents or email CV to vinitagavander@microlabs.in or goahr@microlabs.in, subject: “[Position] – Verna, May 2025.”
- Contact: +91-832-278-3137, goahr@microlabs.in
- Website: www.microlabsltd.com
- Acknowledgement No: LEM2503250
Notes:
- Fraud Alert: Micro Labs does not charge fees. Verify via goahr@microlabs.in or www.microlabsltd.com.
Why Micro Labs?
Micro Labs’ Verna facility is USFDA and MHRA-accredited, producing OSD formulations (tablets, capsules) for cardiology, diabetology, and dermatology. With 14 manufacturing plants and brands like Dolo-650 (350 Crore tablets sold since 2020), Micro Labs leads in generics. Rated 3.8/5 for skill development (Indeed), it offers robust training but scores 3.5/5 for career growth due to competitive promotions.
Benefits include health insurance, transport, and canteen. The roles align with India’s $24.4 billion pharma export market (10% CAGR, Invest India).
Job Positions
1. Production – Granulation/Compression/Coating/Capsule Filling
- Position: Officer, Executive
- Qualification: B.Pharm, M.Pharm
- Experience: 1–8 years
- Vacancies: ~8–12 (estimated)
- Skills:
- Operation of RMG, FBD, Fette/Korsch (compression), Glatt/ACG (coating), AF-90T/Zanasi (capsule filling).
- Knowledge of cGMP, BMR/BPR, process validation, and USFDA/MHRA audits.
- Tasks: Execute granulation, compression, coating, or capsule filling; troubleshoot equipment; ensure compliance; support audits.
- Why Join?: Lead OSD production for global markets; 85% tasks support regulatory compliance.
2. Quality Assurance (QA) – IPQA/BPR/Vendor Qualification
- Position: Sr. Officer, Executive
- Qualification: B.Pharm, M.Pharm
- Experience: 5–8 years
- Vacancies: ~5–8
- Skills:
- Expertise in IPQA, BPR review, vendor qualification, QMS (CAPA, OOS, deviations).
- Familiarity with USFDA/MHRA audits, SAP, and cGMP.
- Tasks: Conduct in-process checks, review BPR, qualify vendors, manage QMS, support audits.
- Why Join?: Ensure quality for regulated markets.
3. Production & Packing
- Position: Technical Staff
- Qualification: HSSC, ITI (AOCP, MMCP), D.Pharm, DME, DEE
- Experience: Freshers (2024 batch)
- Vacancies: ~10–15
- Skills:
- Basic knowledge of blister/bottle packing, cartonator, labeling, or Track & Trace.
- Willingness to learn cGMP and SOPs.
- Tasks: Assist in production/packing, operate equipment (e.g., Uhlmann, CVC), maintain records.
- Why Join?: Kickstart your OSD career with hands-on training.
4. Engineering
- Position: Technician
- Qualification: Diploma (Mechanical, Electrical, Mechatronics)
- Experience: Freshers (2024 batch)
- Vacancies: ~5–8
- Skills:
- Basic knowledge of process maintenance, HVAC, or equipment calibration.
- Familiarity with cGMP (preferred).
- Tasks: Support equipment maintenance, perform basic troubleshooting, ensure uptime.
- Why Join?: Build a foundation in pharma engineering.
Who Can Apply?
- Qualifications: B.Pharm, M.Pharm, HSSC, ITI, D.Pharm, DME, DEE, Diploma (2024 batch for freshers).
- Experience: 0–8 years in OSD formulations (USFDA/MHRA exposure for experienced roles).
- Key Skills:
- Production/QA: Granulation, compression, coating, capsule filling, IPQA, BPR, QMS.
- Packing/Engineering: Blister packing, Track & Trace, HVAC, equipment maintenance.
- Shift adaptability (A/B/C) and strong documentation.
- Preferred: Goan domicile, experience with Fette, Glatt, or SAP; audit-facing roles.
How to Prepare
- Bring Documents: CV (highlight USFDA/MHRA experience for experienced roles), passport-size photo, original mark sheets (2024 batch for freshers), payslips (experienced), PAN/Aadhaar (originals + photocopies).
- Dress Smart: Formal attire (shirt, trousers; avoid casuals).
- Study Up:
- Production/QA: Review RMG, Fette compression, Glatt coating, IPQA, QMS (e.g., CAPA, OOS). Prepare examples (e.g., “How did you resolve a compression defect?”).
- Packing/Engineering: Study blister packing, Track & Trace, or HVAC basics. Discuss willingness to learn (e.g., “I’m eager to master Uhlmann machines”).
- Research Micro Labs’ Verna facility and portfolio (e.g., Dolo-650, cardiology drugs). Visit www.microlabsltd.com.
- Address shift concerns diplomatically (28% rate work-life balance low, AmbitionBox).
- Arrive Early: 8:30 AM for registration; expect 1–2 hour wait (AmbitionBox). GSRTC buses to Verna; parking available.
- Interview: Expect technical (70%) and behavioral (30%) questions; emphasize regulatory exposure (experienced) or eagerness to learn (freshers).
Why Verna, Goa?
Micro Labs’ Verna facility, located in Verna Industrial Estate, is part of Goa’s $1 billion pharma sector (8% CAGR, Invest India). Just 15 km from Panaji via NH-66, it offers 200+ OSD jobs (Naukri) and affordable living (₹12,000/month for 1BHK, AmbitionBox). Company transport from Panaji/Margao ensures connectivity. Ideal for regulatory-focused OSD careers.
Contact Information
- Email: vinitagavander@microlabs.in, goahr@microlabs.in
- Phone: +91-832-278-3137
- Venue/Work Location: Micro Labs Limited, Plot No. S-155-S-159, Phase III, Verna Industrial Estate, Verna, Goa 403722
- Corporate Office: Micro Labs Limited, 27 Race Course Road, Bangalore, Karnataka 560001
- Connect: LinkedIn, Facebook
- Website: www.microlabsltd.com
Apply Now for Micro Labs’ Walk-In on May 10, 2025, in Verna, Goa, to join a USFDA-approved OSD leader! Freshers and experienced candidates welcome!
