Join Swiss Garnier Life Sciences, a global pharmaceutical manufacturer founded in 2006, headquartered in Chennai, Tamil Nadu. With a turnover of ₹350 Cr and USFDA, EUGMP, and ISO 9001:2015 certifications, our Una, Himachal Pradesh facility (50,000 sq.ft.) produces tablets, capsules, liquids, and more for 40+ countries.
We’re hiring for Production (OSD, Liquid), Packing, Documentation, and Analytical Research & Development (AR&D) roles at our Mehatpur plant, targeting candidates with 3-10 years of experience in regulated pharma environments.
Contents
Job Opportunities at Swiss Garnier Life Sciences
We’re seeking skilled professionals for multiple roles in Oral Solid Dosage (OSD) production, packing, liquid manufacturing, and AR&D. All positions are based at our USFDA-compliant facility in Una, a key hub for innovative formulations. Operators must undergo a mandatory trial.
Job Location
- Swiss Garnier Life Sciences, Plot No. 21-22, Industrial Area Mehatpur, Una, Himachal Pradesh – 174315
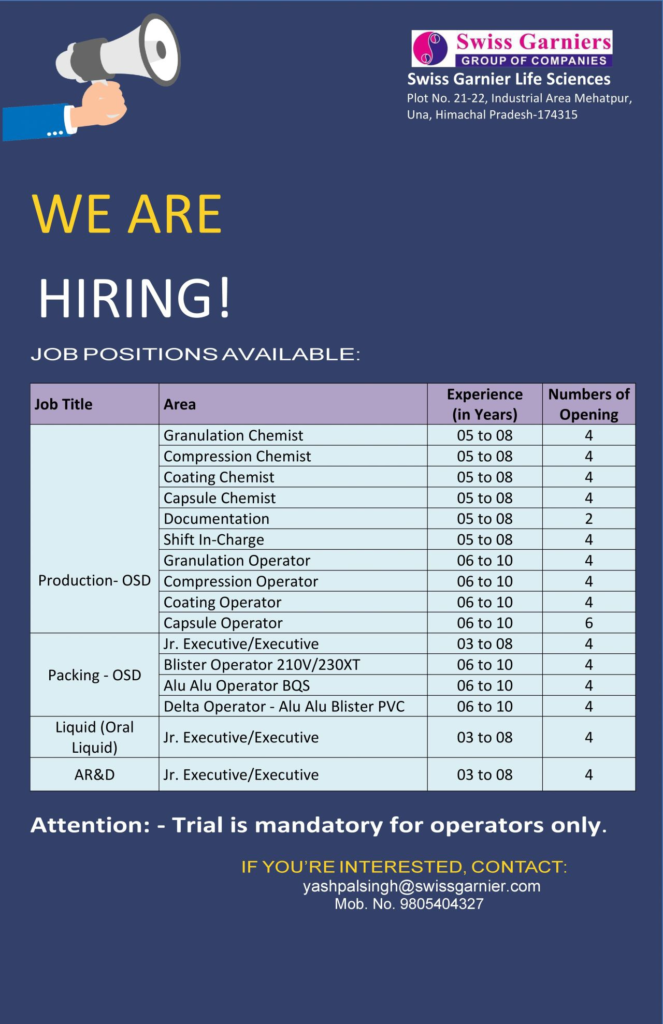
1. Production – Oral Solid Dosage (OSD)
Granulation Chemist:
- Experience: 5-8 years
- Openings: 4
- Responsibilities: Oversee wet/dry granulation, fluid bed drying, and blending; ensure cGMP compliance; document BPRs
- Skills: Expertise in granulation equipment (e.g., RMG, FBD); knowledge of QbD and ICH Q3D
Compression Chemist:
- Experience: 5-8 years
- Openings: 4
- Responsibilities: Manage tablet compression using rotary presses; optimize tooling; ensure tablet quality
- Skills: Proficiency in compression machines (e.g., Cadmach, Sejong); familiarity with USP dissolution
Coating Chemist:
- Experience: 5-8 years
- Openings: 4
- Responsibilities: Conduct film/sugar coating; manage coating parameters; ensure coating uniformity
- Skills: Knowledge of coating systems (e.g., AutoCoater); experience with defect analysis
Capsule Chemist:
- Experience: 5-8 years
- Openings: 4
- Responsibilities: Oversee capsule filling (hard gelatin); ensure fill weight accuracy; manage encapsulation
- Skills: Expertise in capsule fillers (e.g., ACG, Bosch); knowledge of pellet filling
Granulation Operator:
- Experience: 6-10 years
- Openings: 4
- Responsibilities: Operate RMG, FBD, and blenders; perform cleaning/validation; complete BPRs
- Skills: Hands-on granulation experience; cGMP and SOP adherence
Compression Operator:
- Experience: 6-10 years
- Openings: 4
- Responsibilities: Operate rotary compression machines; perform in-process checks; maintain tooling
- Skills: Proficiency in tablet presses; knowledge of weight variation control
Coating Operator:
- Experience: 6-10 years
- Openings: 4
- Responsibilities: Operate coating pans; monitor spray rates; ensure defect-free tablets
- Skills: Experience with coating equipment; familiarity with cleaning validation
Capsule Operator:
- Experience: 6-10 years
- Openings: 6
- Responsibilities: Operate capsule filling machines; perform weight checks; document processes
- Skills: Hands-on encapsulation; knowledge of cGMP documentation
Shift In-Charge:
- Experience: 5-8 years
- Openings: 4
- Responsibilities: Supervise OSD production shifts; ensure schedule adherence; manage team of 10-15
- Skills: Leadership in regulated plants; expertise in BMR/BPR and QMS
2. Production – Liquid (Oral Liquid)
Jr. Executive/Executive:
- Experience: 3-8 years
- Openings: 4
- Responsibilities: Oversee oral liquid manufacturing (suspensions, syrups); manage mixing/filling; ensure cGMP
- Skills: Knowledge of liquid filling lines; experience with USP standards
3. Packing – Oral Solid Dosage (OSD)
Jr. Executive/Executive:
- Experience: 3-8 years
- Openings: 4
- Responsibilities: Supervise OSD packing lines; ensure track & trace compliance; manage BPCR
- Skills: Expertise in blister/Alu-Alu packing; knowledge of serialization
Blister Operator (210V/230XT):
- Experience: 6-10 years
- Openings: 4
- Responsibilities: Operate 210V/230XT blister machines; perform changeovers; ensure packing quality
- Skills: Proficiency in Pam-Pac/Elmach blister machines; cGMP documentation
Alu Alu Operator (BQS):
- Experience: 6-10 years
- Openings: 4
- Responsibilities: Operate BQS Alu-Alu machines; monitor sealing; complete logbooks
- Skills: Hands-on Alu-Alu packing; knowledge of leak testing
Delta Operator (Alu-Alu/Blister/PVC):
- Experience: 6-10 years
- Openings: 4
- Responsibilities: Operate Delta blister/Alu-Alu machines; ensure PVC film quality; perform in-process checks
- Skills: Experience with Delta machines; familiarity with track & trace
4. Documentation
- Position: Documentation Specialist
- Experience: 5-8 years
- Openings: 2
- Responsibilities: Prepare/review SOPs, BMRs, BPRs; ensure 21CFR Part 11 compliance; support audits
- Skills: Expertise in QMS documentation; knowledge of USFDA/MHRA requirements
5. Analytical Research & Development (AR&D)
- Jr. Executive/Executive:
- Experience: 3-8 years
- Openings: 4
- Responsibilities: Develop/validate analytical methods for OSD/liquids; perform HPLC/GC testing; support ANDA filings
- Skills: Proficiency in HPLC; knowledge of ICH Q2(R1) and GLP
Qualifications and Experience
| Area | Job Title | Experience | Openings | Key Skills |
|---|---|---|---|---|
| Production (OSD) | Granulation Chemist | 5-8 years | 4 | Granulation, cGMP, QbD |
| Production (OSD) | Compression Chemist | 5-8 years | 4 | Compression, USP, Tooling |
| Production (OSD) | Coating Chemist | 5-8 years | 4 | Coating, Defect Analysis |
| Production (OSD) | Capsule Chemist | 5-8 years | 4 | Encapsulation, Pellet Filling |
| Production (OSD) | Granulation Operator | 6-10 years | 4 | RMG, FBD, BPR |
| Production (OSD) | Compression Operator | 6-10 years | 4 | Tablet Press, Weight Control |
| Production (OSD) | Coating Operator | 6-10 years | 4 | Coating Pan, Validation |
| Production (OSD) | Capsule Operator | 6-10 years | 6 | Capsule Filler, cGMP |
| Production (OSD) | Shift In-Charge | 5-8 years | 4 | Leadership, QMS, BMR |
| Production (Liquid) | Jr. Executive/Executive | 3-8 years | 4 | Liquid Filling, USP |
| Packing (OSD) | Jr. Executive/Executive | 3-8 years | 4 | Blister, Serialization |
| Packing (OSD) | Blister Operator (210V/230XT) | 6-10 years | 4 | Pam-Pac, Leak Testing |
| Packing (OSD) | Alu Alu Operator (BQS) | 6-10 years | 4 | BQS, Logbooks |
| Packing (OSD) | Delta Operator (Alu-Alu/Blister/PVC) | 6-10 years | 4 | Delta, Track & Trace |
| Documentation | Documentation Specialist | 5-8 years | 2 | SOPs, 21CFR Part 11 |
| AR&D | Jr. Executive/Executive | 3-8 years | 4 | HPLC, ICH Q2(R1), ANDA |
Why Join Swiss Garnier Life Sciences?
Swiss Garnier, with 200+ employees, exports to 40+ countries and holds a 3.2/5 AmbitionBox rating for work-life balance. Our Una facility, established in 2006, is renowned for tablet manufacturing and cGMP compliance. Employees value regulatory exposure but note management challenges. With four facilities and a focus on innovation, Swiss Garnier offers a platform for growth in Himachal Pradesh’s pharma hub.
Key Benefits
- Work in a USFDA/EUGMP-compliant facility
- Contribute to generics for 40+ countries
- Gain expertise in OSD, liquids, and HPLC
- Join a 19-year legacy with ₹350 Cr turnover
- Access opportunities in a growing CDMO
How to Apply
Email your CV to yashpalsingh@swissgarnier.com with subject “Application for [Job Title] – Una”. Include:
- Educational certificates (ITI, Diploma, B.Sc., B.Pharm, M.Pharm, M.Sc.)
- Experience letters and last 3 months’ payslips
- Aadhar and PAN cards
- CTC and notice period
- Preparation:
- Chemists/Executives: Review granulation, compression, coating, or HPLC; prepare for cGMP questions
- Operators: Be ready for equipment-specific trials (e.g., RMG, Pam-Pac, Delta); study SOPs
- Documentation: Understand QMS and 21CFR Part 11
- Note: Operators must pass a mandatory trial. Swiss Garnier does not charge recruitment fees; verify communications via @swissgarnier.com emails or +91 9805404327.
About Swiss Garnier Life Sciences
Swiss Garnier operates four manufacturing facilities, with Una specializing in tablets, capsules, and liquids. Incorporated in 2006, we’ve grown into a global CDMO with cGMP excellence. Our mission is to deliver innovative formulations while navigating complex regulatory landscapes.
Our Mission
- Produce high-quality generics for global markets
- Uphold USFDA/EUGMP standards
- Foster innovation in pharmaceutical manufacturing
Apply urgently to yashpalsingh@swissgarnier.com or contact +91 9805404327 to join Swiss Garnier Life Sciences in Una. Shape the future of pharmaceutical manufacturing
