Covalent Laboratories Private Limited, a USFDA, EU-GMP, WHO-GMP, and ISO 14001:2004 certified pharmaceutical company specializing in Cephalosporin APIs, is hosting a walk-in interview for Production, Safety, Research & Development (R&D), and Effluent Treatment Plant (ETP) roles at our facility in Gundlamachnoor, Sangareddy, Telangana. Join our dynamic team to contribute to high-quality pharmaceutical manufacturing at a globally recognized organization!
Contents
Event Details
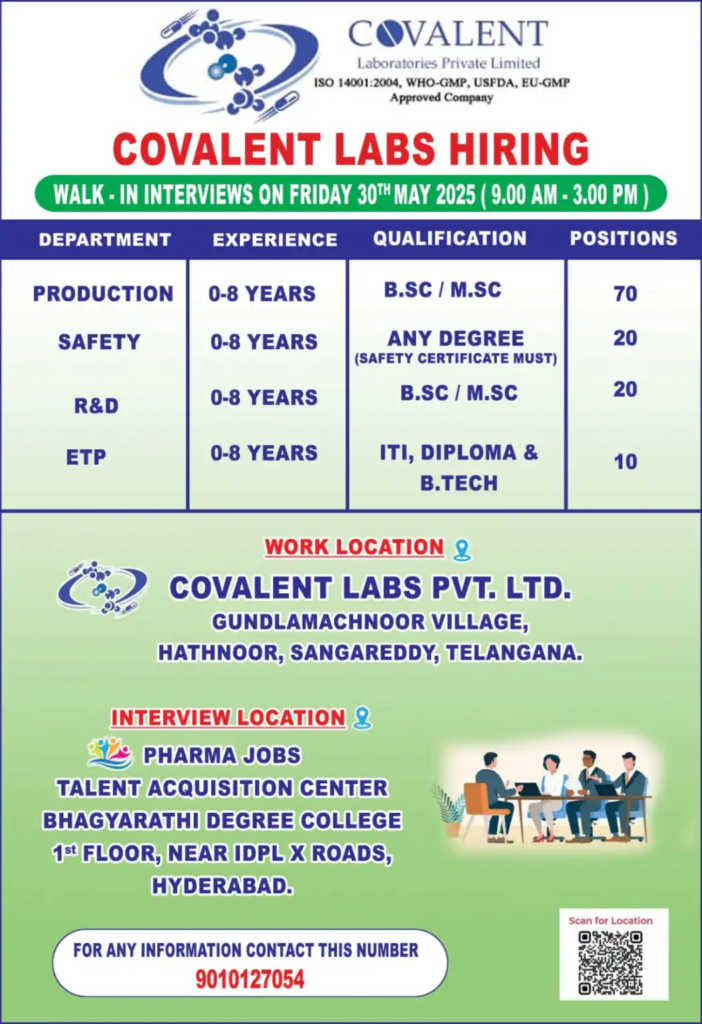
Date: Friday, May 30, 2025
Time: 9:00 AM to 3:00 PM
Interview Venue: Bhagyarthi Degree College, 1st Floor, Near IDPL X Roads, Hyderabad, Telangana
Work Location: Covalent Laboratories Pvt. Ltd., Gundlamachnoor Village, Hathnoor Mandal, Sangareddy District, Telangana 502296
Contact: +91 9010127054
Apply: Attend walk-in or scan the QR code (if provided in the job posting) for registration. Email resumes to hr@covalentlab.com if unable to attend.
Website: www.covalentlab.com
Open Positions
We’re hiring for Production, Safety, R&D, and ETP departments with a total of 120 openings. Below are the details:
| Department | Positions | Qualification | Experience | No. of Openings | Key Responsibilities |
|---|---|---|---|---|---|
| Production | Chemist, Sr. Chemist, Operator, Executive | B.Sc., M.Sc. | 0-8 Years | 70 | API production, GMP compliance, equipment operation |
| Safety | Safety Officer, Executive | Any Degree + Safety Cert. | 0-8 Years | 20 | Hazard assessment, safety protocols, compliance |
| R&D | Chemist, Research Associate | B.Sc., M.Sc. (Org. Chem.) | 0-8 Years | 10 | Method development, API synthesis, HPLC/GC analysis |
| ETP | Operator, Chemist, Executive | ITI, Diploma, B.Tech | 0-8 Years | 20 | Effluent treatment, ZLD operations, troubleshooting |
Production
- Roles: Chemist, Senior Chemist, Operator, Executive
- Responsibilities:
- Operate equipment for Cephalosporin API manufacturing (e.g., reactors, centrifuges, dryers).
- Ensure compliance with cGMP and maintain batch records.
- Troubleshoot production issues and support process optimization.
- Preferences: Experience in USFDA/EU-GMP-regulated API plants, knowledge of GMP documentation.
Safety
- Roles: Safety Officer, Executive
- Responsibilities:
- Assess and document workplace hazards, risks, and controls.
- Implement safety protocols and ensure regulatory compliance (OSHA, local standards).
- Conduct safety training and audits.
- Requirements: Safety certificate (Diploma/PG in Industrial Safety) mandatory.
- Preferences: Pharma industry experience, familiarity with API manufacturing safety.
Research & Development (R&D)
- Roles: Chemist, Research Associate
- Responsibilities:
- Develop and validate analytical methods for APIs using HPLC, GC, or UPLC.
- Support API synthesis, process optimization, and scale-up activities.
- Maintain GLP-compliant documentation and lab records.
- Preferences: Hands-on experience with HPLC/GC, knowledge of LCMS, and organic chemistry expertise.
Effluent Treatment Plant (ETP)
- Roles: Operator, Chemist, Executive
- Responsibilities:
- Operate ETP systems, including Multiple Effect Evaporators (MEE) and Zero Liquid Discharge (ZLD).
- Monitor effluent quality and ensure compliance with environmental regulations.
- Perform routine maintenance and troubleshoot ETP equipment.
- Preferences: Experience in pharma ETP operations, familiarity with solvent recovery systems.
Candidate Requirements
- Qualifications:
- Production & R&D: B.Sc., M.Sc. (Chemistry, Organic Chemistry).
- Safety: Any Degree with Safety Certificate (Diploma/PG in Industrial Safety).
- ETP: ITI (Fitter, Electrical), Diploma (Chemical/Mechanical), B.Tech (Chemical).
- Experience: 0-8 years (freshers and experienced candidates welcome).
- Skills:
- Production: API manufacturing, GMP, equipment handling.
- Safety: Hazard analysis, safety training, compliance.
- R&D: HPLC, GC, method development, GLP.
- ETP: Effluent treatment, ZLD, troubleshooting.
- Preferences:
- Experience in USFDA/EU-GMP-regulated API plants.
- Willingness to work in rotational shifts.
- Strong communication and documentation skills.
- Work Location: Gundlamachnoor, Sangareddy, Telangana (on-site).
Documents Required
- Updated resume
- Educational certificates/mark sheets
- Safety certificate (for Safety roles)
- Last 3 months’ payslips (if applicable)
- Recent increment letter (if applicable)
- Copies of Aadhar and PAN card
- 2 passport-size photographs
Recruitment Process
- Registration: Register at the venue or via QR code (if provided).
- Screening: Initial resume and document review.
- Technical Interview: Assess domain-specific skills (e.g., API production, HPLC, safety protocols).
- HR Interview: Evaluate shift adaptability and cultural fit.
Why Join Covalent Laboratories?
Covalent Laboratories Pvt. Ltd., established in 2002, is a leading Cephalosporin API manufacturer with USFDA, EU-GMP, and WHO-GMP certifications. Headquartered in Hyderabad, we are rated 3.1/5 on AmbitionBox, with job security at 3.2/5. Our Gundlamachnoor facility offers state-of-the-art infrastructure, benefits like ESI, PF, gratuity, and subsidized canteen food, and a collaborative environment. With a strong export presence in regulated markets, we provide career growth opportunities for freshers and experienced professionals. Learn more at Covalent Laboratories’ website or follow us on LinkedIn.
How to Apply
Attend the walk-in interview on May 30, 2025, from 9:00 AM to 3:00 PM at Bhagyarthi Degree College, 1st Floor, Near IDPL X Roads, Hyderabad. Bring the required documents listed above. Register in advance by scanning the QR code (if available) or contacting +91 9010127054. Candidates unable to attend can email their resume to hr@covalentlab.com, with the subject line “Application for [Department] – [Your Name]”. For updates, connect via LinkedIn.



I worked from Nester pharma company faridaba I have experience 2 years work