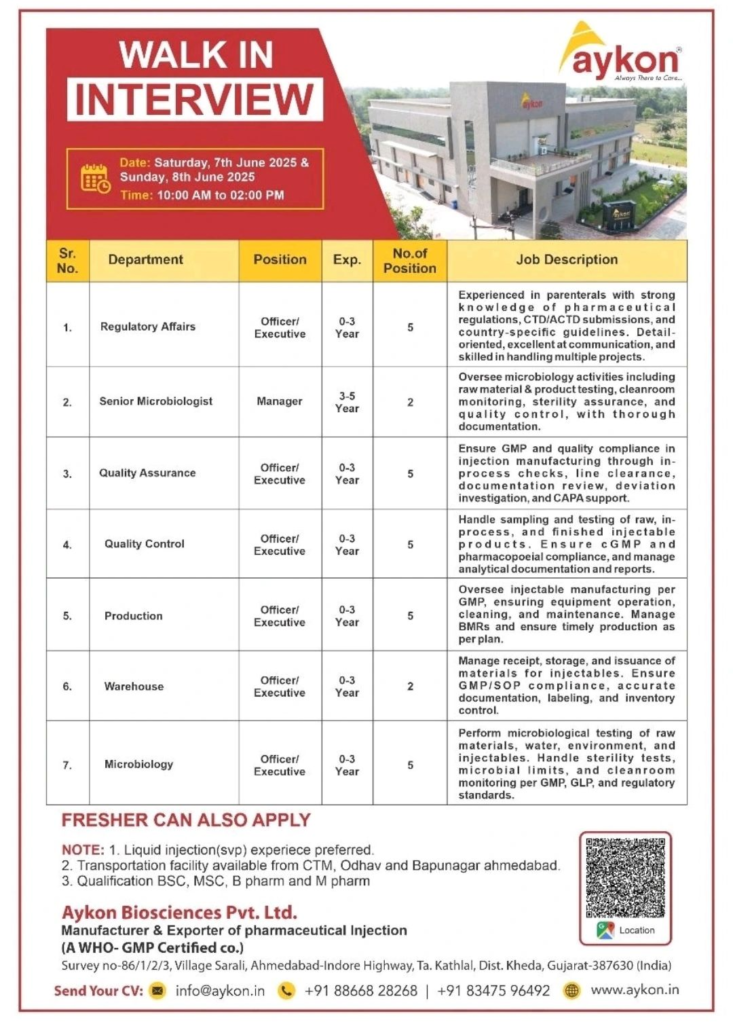
Join Aykon Biosciences Pvt. Ltd., a WHO-GMP certified manufacturer and exporter of pharmaceutical injections, for exciting career opportunities in the pharmaceutical industry. We are hosting walk-in interviews on June 7th and 8th, 2025, at our facility in Sarali, Gujarat, for multiple roles in Regulatory Affairs, Microbiology, Quality Assurance, Quality Control, Production, and Warehouse. Freshers and experienced professionals are welcome to apply for these rewarding healthcare careers.
Contents
Why Choose Aykon Biosciences?
Aykon Biosciences is committed to delivering high-quality injectable pharmaceuticals globally. Our Sarali facility offers a modern, GMP-compliant work environment, fostering professional growth and innovation. With a focus on quality and compliance, we provide opportunities to work on cutting-edge parenteral manufacturing processes. We also offer transportation facilities from CTM, Odhav, and Bapunagar in Ahmedabad to make your interview experience seamless.
Open Positions in Pharmaceutical Manufacturing
We are seeking talented individuals for various roles in our pharmaceutical operations. Below is a detailed overview of the open positions:
| Sr. No. | Department | Position | Experience (Years) | No. of Positions |
|---|---|---|---|---|
| 1 | Regulatory Affairs | Officer/Executive | 0-3 | 5 |
| 2 | Microbiology | Senior Microbiologist/Manager | 3-5 | 2 |
| 3 | Quality Assurance | Officer/Executive | 0-3 | 5 |
| 4 | Quality Control | Officer/Executive | 0-3 | 5 |
| 5 | Production | Officer/Executive | 0-3 | 5 |
| 6 | Warehouse | Officer/Executive | 0-3 | 2 |
| 7 | Microbiology | Officer/Executive | 0-3 | 5 |
Job Descriptions
- Regulatory Affairs (Officer/Executive): Handle CTD/ACTD submissions, ensure compliance with country-specific pharmaceutical regulations, and manage multiple projects with strong communication and detail-oriented skills.
- Senior Microbiologist (Manager): Oversee microbiology activities, including raw material and product testing, cleanroom monitoring, sterility assurance, and quality control, with meticulous documentation.
- Quality Assurance (Officer/Executive): Ensure GMP compliance in injectable manufacturing through in-process checks, line clearance, deviation investigation, CAPA support, and documentation review.
- Quality Control (Officer/Executive): Perform sampling and testing of raw, in-process, and finished injectable products, ensuring cGMP and pharmacopoeial compliance while managing analytical documentation.
- Production (Officer/Executive): Manage injectable manufacturing per GMP standards, oversee equipment operation, cleaning, maintenance, and ensure timely production with accurate Batch Manufacturing Records (BMRs).
- Warehouse (Officer/Executive): Manage receipt, storage, and issuance of materials for injectables, ensuring GMP/SOP compliance, accurate labeling, and inventory control.
- Microbiology (Officer/Executive): Conduct microbiological testing of raw materials, water, environment, and injectables, including sterility tests, microbial limits, and cleanroom monitoring per GMP and GLP standards.
Interview Details
- Date: Saturday, June 7, 2025, and Sunday, June 8, 2025
- Time: 10:00 AM to 2:00 PM
- Venue: Aykon Biosciences Pvt. Ltd., Survey No. 86/1/2/3, Village Sarali, Ahmedabad-Indore Highway, Ta. Kathlal, Dist. Kheda, Gujarat-387630, India
- Work Location: Same as interview venue
How to Apply
Attend the walk-in interviews with your updated resume and relevant documents. For pre-registration or queries, send your CV to info@aykon.in or contact our HR team at +91 88668 28268 or +91 83475 96492. Visit www.aykon.in for more information about our company and mission.
Qualifications
Eligible candidates should possess one of the following qualifications:
- B.Sc
- M.Sc
- B.Pharm
- M.Pharm
Freshers with relevant qualifications are encouraged to apply, particularly those with an interest in liquid injection (SVP) processes.
Special Notes
- Preference: Candidates with experience in liquid injection (Small Volume Parenterals – SVP) are preferred.
- Transportation: Complimentary transportation will be provided from CTM, Odhav, and Bapunagar in Ahmedabad to the interview venue.
- Career Growth: Aykon Biosciences offers a supportive environment for career advancement, with opportunities to work on innovative pharmaceutical projects.
Why Sarali, Gujarat?
Located on the Ahmedabad-Indore Highway, our Sarali facility is a hub for pharmaceutical excellence. With WHO-GMP certification, it provides a state-of-the-art workplace for professionals passionate about healthcare innovation. Join a team dedicated to improving global health through high-quality injectable products.
Prepare for Your Interview
To maximize your chances of success:
- Bring an updated resume highlighting your qualifications and experience.
- Be ready to discuss your knowledge of GMP, GLP, and pharmaceutical regulations.
- Research Aykon Biosciences’ contributions to the injectable pharmaceutical industry.
This is your opportunity to join a leading pharmaceutical company committed to quality and innovation. We look forward to meeting you at the walk-in interviews on June 7th and 8th, 2025, at Aykon Biosciences Pvt. Ltd.!


