Pharmaceutical testing ensures drug safety and efficacy. In 2025, rigorous quality control is vital for tablet manufacturing. These tests confirm that medications meet global standards, protecting patients worldwide.
Why Drug Quality Control Matters
Drug quality control prevents defective medications from reaching consumers. It ensures tablets are safe, effective, and reliable. By conducting these tests, manufacturers comply with regulations and maintain trust in the pharmaceutical industry.
Key Tests for Tablet Manufacturing
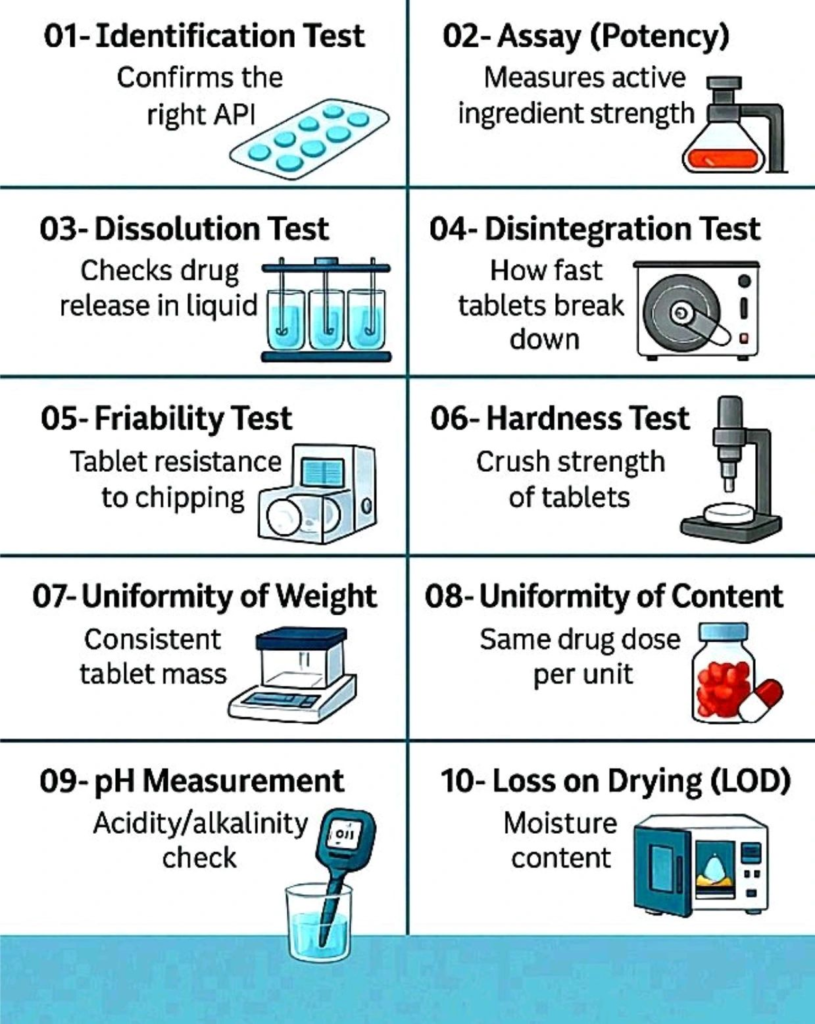
Here are 10 critical tests for ensuring tablet quality:
1. Identification Test
Purpose
Confirms the correct active pharmaceutical ingredient (API).
Importance
Ensures the drug contains the right API, avoiding mix-ups during production.
2. Assay (Potency) Test
Purpose
Measures the strength of the active ingredient.
Importance
Verifies the drug’s potency for effective treatment, a key step in quality control.
3. Dissolution Test
Purpose
Checks how the drug releases in liquid.
Importance
Ensures the tablet dissolves properly for absorption in the body, impacting efficacy.
4. Disintegration Test
Purpose
Measures how fast tablets break down.
Importance
Confirms the tablet disintegrates quickly for proper drug release in the stomach.
5. Friability Test
Purpose
Tests tablet resistance to chipping.
Importance
Ensures tablets withstand handling without breaking, maintaining quality during transport.
6. Hardness Test
Purpose
Measures the crush strength of tablets.
Importance
Ensures tablets are durable but not too hard to dissolve effectively.
7. Uniformity of Weight
Purpose
Checks consistent tablet mass.
Importance
Ensures each tablet has the same weight, indicating uniform production quality.
8. Uniformity of Content
Purpose
Verifies the same drug dose per unit.
Importance
Guarantees consistent dosage in each tablet, critical for patient safety.
9. pH Measurement
Purpose
Checks acidity/alkalinity levels.
Importance
Ensures the drug’s pH is safe for consumption and stability.
10. Loss on Drying (LOD)
Purpose
Measures moisture content.
Importance
Confirms low moisture to prevent degradation, ensuring a longer shelf life.
Summary Table of Tests
| Test | Purpose | Key Benefit |
|---|---|---|
| Identification Test | Confirms right API | Avoids production errors |
| Assay (Potency) | Measures active ingredient | Ensures effective treatment |
| Dissolution Test | Checks drug release | Ensures proper absorption |
| Disintegration Test | Measures breakdown speed | Confirms quick drug release |
| Friability Test | Tests resistance to chipping | Maintains tablet integrity |
Benefits of Rigorous Testing
- Patient Safety: Prevents harmful defects in medications.
- Regulatory Compliance: Meets standards set by agencies like the FDA.
- Brand Trust: Builds confidence in pharmaceutical products.
Conclusion
Pharmaceutical testing is crucial for drug quality in 2025. From potency to moisture content, these tests ensure tablets are safe and effective. Manufacturers must prioritize quality control to protect patients and uphold industry standards.
