Join Aizant Drug Research Solutions Pvt. Ltd., a leading integrated drug development solutions provider in Hyderabad, established in 2005 with USFDA and EU-GMP approvals. Attend our walk-in interview on June 13, 2025, for roles in Quality Assurance (QA) and Quality Control (QC).
Contents
Event Details
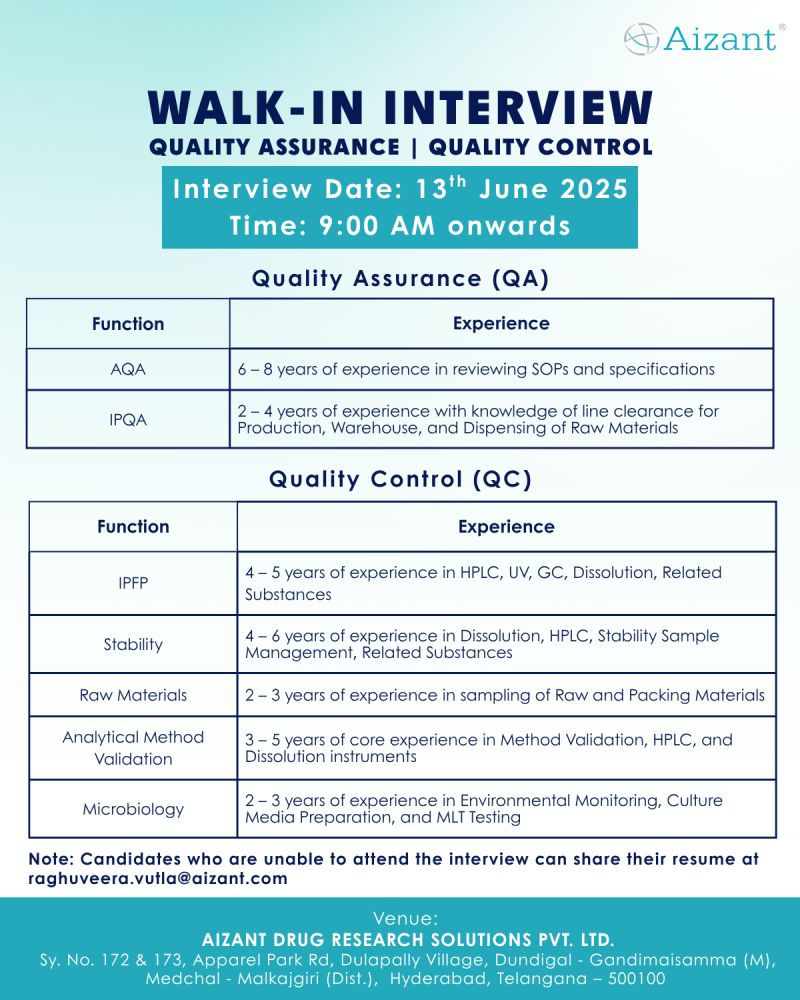
Date: June 13, 2025 (Friday)
Time: 9:00 AM onwards
Venue: Aizant Drug Research Solutions Pvt. Ltd., Sy. No. 172 & 173, Apparel Park Rd, Dulapally Village, Dundigal-Gandimaisamma (M), Medchal-Malkajgiri, Hyderabad, Telangana-500100
Apply: Unable to attend? Email resume to raghuveera.vutla@aizant.com with the subject indicating the role (e.g., “IPQA Application”)
Work Location: Hyderabad, Telangana
Job Opportunities
Aizant, rated 3.8/5 for skill development by 340+ employees on AmbitionBox, seeks experienced professionals for QA and QC roles in our formulation CDMO and CRO operations.
Quality Assurance (QA)
Analytical Quality Assurance (AQA)
- Experience: 6–8 years
- Responsibilities: Review SOPs, specifications, and analytical documents in a cGMP environment
- Skills: Expertise in analytical quality assurance and regulatory compliance
- Positions: 2
In-Process Quality Assurance (IPQA)
- Experience: 2–4 years
- Responsibilities: Provide line clearance for production, warehouse, and raw material dispensing
- Skills: Knowledge of IPQA processes and cGMP standards
- Positions: 3
Quality Control (QC)
In-Process Finished Product (IPFP)
- Experience: 4–5 years
- Responsibilities: Perform HPLC, UV, GC, dissolution, and related substances testing
- Skills: Proficiency in analytical instruments and QC testing
- Positions: 2
Stability
- Experience: 4–6 years
- Responsibilities: Manage stability sample testing, dissolution, HPLC, and related substances analysis
- Skills: Expertise in stability studies and analytical techniques
- Positions: 2
Raw Materials
- Experience: 2–3 years
- Responsibilities: Conduct sampling and testing of raw and packing materials
- Skills: Knowledge of material sampling and QC processes
- Positions: 3
Analytical Method Validation
- Experience: 3–5 years
- Responsibilities: Perform method validation using HPLC and dissolution instruments
- Skills: Core expertise in analytical method validation
- Positions: 2
Microbiology
- Experience: 2–3 years
- Responsibilities: Conduct environmental monitoring, culture media preparation, and MLT testing
- Skills: Proficiency in microbiological testing and GLP
- Positions: 2
Eligibility Criteria
- Experience in pharmaceutical formulation (OSD/injectables) with cGMP-compliant facilities
- Candidates must be open to shift duties
- Prior experience in USFDA/EU-GMP regulated plants preferred
- Hyderabad-based or willing to relocate
Documents to Carry
Bring original and photocopies of:
- Updated resume
- Educational certificates
- Experience certificates
- Last three months’ payslips
- Aadhaar and PAN card
- Passport-size photographs
Why Join Aizant?
Aizant, with 700+ employees and a 3.7/5 work-life balance rating, offers a collaborative culture and skill development opportunities. Our Hyderabad facility supports global clients in NMEs, generics, and OTCs. Learn more at Aizant Careers.
How to Prepare
- Align your experience with the role requirements.
- Bring all required documents to the venue.
- Contact Aizant for directions.
Verified by Trusted HRs
The post is released by the Aizant LinkedIn page. Click here to visit the post
Contact Us
For queries, email raghuveera.vutla@aizant.com or call +91-40-2308-1234. Join Aizant Drug Research Solutions in Hyderabad to advance your pharmaceutical career!


