Eugia Pharma Specialities Ltd., a wholly-owned subsidiary of Aurobindo Pharma Limited, is hosting a walk-in interview for engineering roles at our USFDA-approved injectable manufacturing facility in Pashamylaram, Hyderabad. Join our team to contribute to high-impact therapies in oncology, anti-infectives, and complex injectables, serving patients in over 120 countries
About Eugia Pharma Specialities
Established in 2013, Eugia Pharma Specialities Ltd. is a leading manufacturer of general injectables, oncology, ophthalmics, and hormonal products, with a global presence and over 1,151 employees. Our Unit-III facility at Pashamylaram is cGMP-compliant but recently faced USFDA observations for data integrity lapses, which we are actively addressing.
Rated 4.1/5 on AmbitionBox for job security (4.5/5), employees praise punctuality and OT benefits, though work-life balance (3.8/5) and career growth (3.6/5) are mixed due to management challenges.
Job Opportunities at Unit-III (Engineering – Injectable Manufacturing)
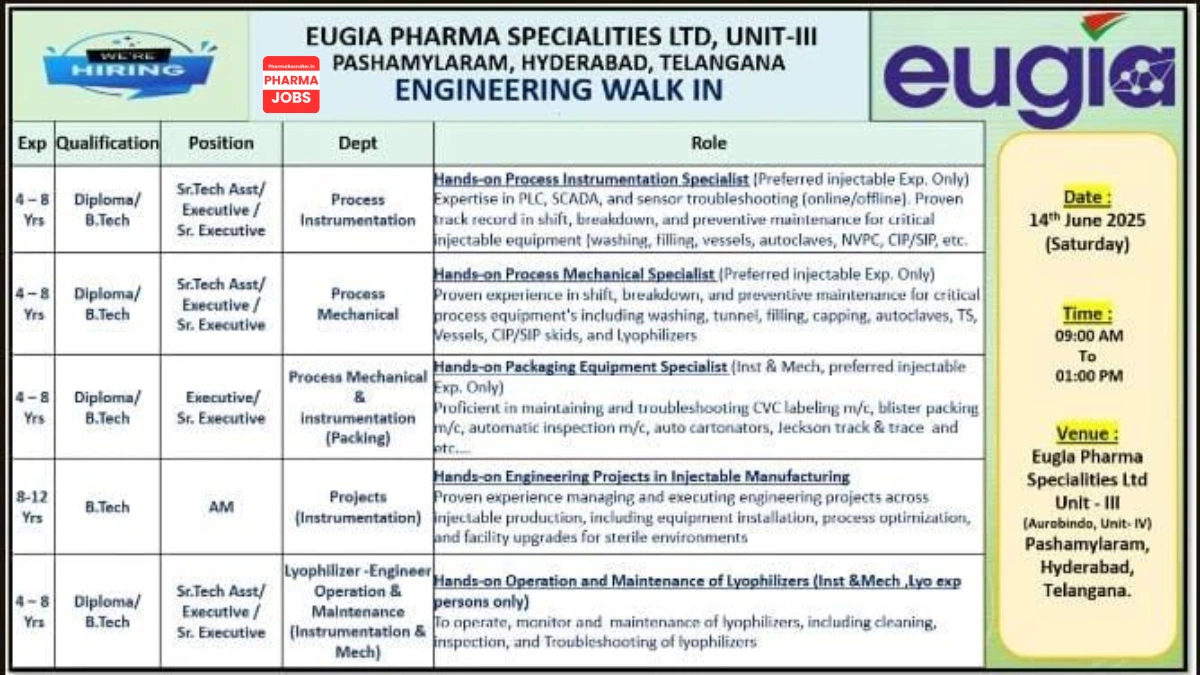
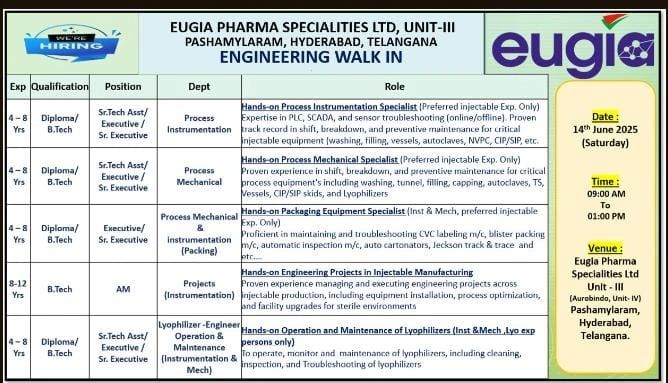
We are hiring for roles in Process Instrumentation, Process Mechanical, Packaging, Projects, and Lyophilizer Operation & Maintenance. Candidates with injectable manufacturing experience and immediate availability are preferred. All roles require 4-12 years of experience in API/injectable pharma environments.
Senior Technical Assistant / Executive / Senior Executive – Process Instrumentation
- Qualification: Diploma / B.Tech (Instrumentation / Electronics)
- Experience: 4-8 years in injectable manufacturing
- Department: Engineering
- Responsibilities:
- Perform shift, breakdown, and preventive maintenance for critical injectable equipment (washing, filling, vessels, autoclaves, NVPC, CIP/SIP)
- Troubleshoot PLC, SCADA, and sensors (online/offline)
- Ensure GMP compliance and accurate maintenance documentation
- Required Skills:
- Hands-on expertise in process instrumentation for injectables
- Proficiency in PLC, SCADA, and sensor troubleshooting
- Knowledge of GMP and sterile equipment maintenance
- Vacancies: Multiple
Senior Technical Assistant / Executive / Senior Executive – Process Mechanical
- Qualification: Diploma / B.Tech (Mechanical)
- Experience: 4-8 years in injectable manufacturing
- Department: Engineering
- Responsibilities:
- Conduct shift, breakdown, and preventive maintenance for equipment (washing, tunnel, filling, capping, autoclaves, TS, vessels, CIP/SIP skids, lyophilizers)
- Support equipment qualification and process optimization
- Maintain GMP-compliant documentation
- Required Skills:
- Hands-on experience with injectable process equipment
- Expertise in mechanical maintenance and troubleshooting
- Familiarity with GMP and sterile manufacturing
- Vacancies: Multiple
Executive / Senior Executive – Process Mechanical & Instrumentation (Packing)
- Qualification: Diploma / B.Tech (Mechanical / Instrumentation)
- Experience: 4-8 years in injectable manufacturing
- Department: Engineering
- Responsibilities:
- Maintain and troubleshoot packaging equipment (CVC labeling, blister packing, automatic inspection, auto cartonators, Leckson track & trace)
- Perform preventive and breakdown maintenance
- Ensure GMP compliance and accurate records
- Required Skills:
- Proficiency in packaging equipment maintenance (mechanical and instrumentation)
- Experience with injectable packaging systems
- Knowledge of GMP and QMS
- Vacancies: Multiple
Assistant Manager – Projects (Instrumentation)
- Qualification: B.Tech (Instrumentation / Electronics)
- Experience: 8-12 years in injectable manufacturing
- Department: Engineering
- Responsibilities:
- Manage engineering projects for injectable production (equipment installation, process optimization, facility upgrades)
- Oversee validation, qualification, and regulatory compliance
- Coordinate with cross-functional teams for sterile environment upgrades
- Required Skills:
- Proven project management experience in injectable manufacturing
- Expertise in instrumentation and sterile facility upgrades
- Knowledge of GMP, USFDA, and project execution
- Vacancies: Limited
Senior Technical Assistant / Executive / Senior Executive – Lyophilizer Engineer (Operation & Maintenance)
- Qualification: Diploma / B.Tech (Mechanical / Instrumentation)
- Experience: 4-8 years in lyophilizer operation and maintenance (injectable manufacturing)
- Department: Engineering
- Responsibilities:
- Operate, monitor, and maintain lyophilizers (cleaning, inspection, troubleshooting)
- Perform preventive and breakdown maintenance (instrumentation and mechanical)
- Ensure GMP compliance and accurate documentation
- Required Skills:
- Hands-on experience with lyophilizer operation and maintenance
- Proficiency in mechanical and instrumentation troubleshooting
- Knowledge of GMP and sterile processes
- Vacancies: Multiple
Walk-In Interview Details
- Date: 14th June 2025 (Saturday)
- Time: 9:00 AM – 1:00 PM
- Venue: Eugia Pharma Specialities Ltd., Unit-III (Aurobindo Unit-IV), Plot No. 4, 34 to 48, Phase-III, EPIP, APIIC, Pashamylaram, Patancheru (Mandal), Sangareddy, Hyderabad, Telangana – 502329
- Contact: hr@eugiapharma.com
- Phone: Not provided; email for queries
- Website: www.eugiapharma.com
Documents to Bring
- Updated resume with passport-sized photograph
- Copies of all educational certificates and mark sheets
- Experience certificates and relieving letters
- Last 3 months’ payslips and increment letter
- Aadhaar Card and PAN Card
How to Apply
Attend the walk-in interview with the required documents. If unable to attend, email your CV to hr@eugiapharma.com with the subject “Application for [Position Name] – Unit-III – June 2025.” Only candidates with injectable experience are eligible.
Why Join Eugia Pharma Specialities?
Eugia offers a platform to work in a USFDA-approved injectable facility with global reach, producing high-impact therapies. Employees benefit from job security (4.5/5) and OT benefits, though concerns include management practices and canteen quality.
The company is addressing USFDA observations to strengthen compliance. Join a team driving innovation in complex injectables
Why Pashamylaram?
Pashamylaram, an industrial hub near Hyderabad, hosts Eugia’s advanced injectable facility, offering career stability in a pharmaceutical hotspot. The location is accessible but may require commuting.
Important Notes
- Eligibility: Only candidates with 4-12 years of injectable manufacturing experience (lyophilizer roles require specific lyo experience). Diploma/B.Tech graduates only. Male candidates preferred due to shift operations.
- Disclaimer: Eugia Pharma Specialities does not charge fees for job applications. Use only official email hr@eugiapharma.com. Beware of fraudulent offers.
- Note: Candidates interviewed in the last 6 months need not reapply. The facility is under USFDA scrutiny; candidates should be prepared for rigorous compliance standards.
Don’t miss this opportunity to advance your career with Eugia Pharma Specialities! Attend our walk-in interview on 14th June 2025 at Unit-III, Pashamylaram, and contribute to world-class injectable manufacturing!