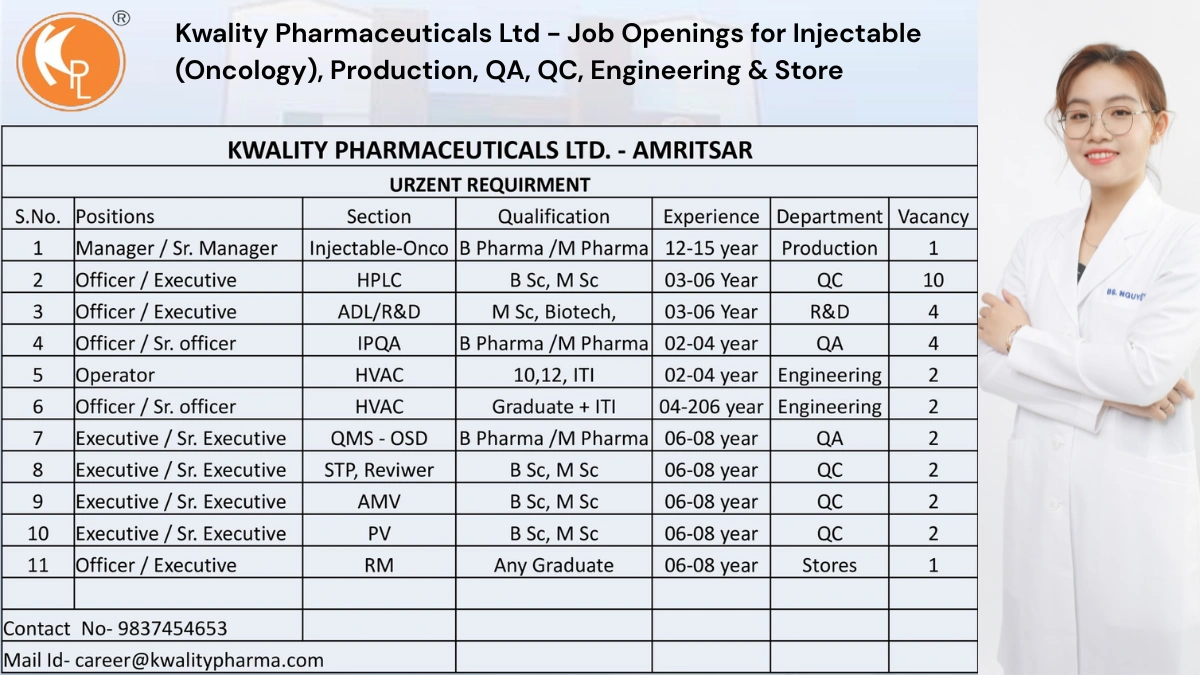
Kwality Pharmaceuticals Ltd., a leading manufacturer of pharmaceutical formulations since 1993, is urgently hiring for multiple roles at its WHO-GMP-certified facility in Amritsar, Punjab. Specializing in injectables (oncology), oral solids, and liquid formulations, Kwality serves domestic and international markets with a commitment to quality. Join our team to advance your career in a dynamic pharmaceutical environment!
About Kwality Pharmaceuticals Ltd.
Headquartered at Village Nag Kalan, Majitha Road, Amritsar, Kwality Pharmaceuticals operates two WHO-GMP facilities in Amritsar and Jassur (Himachal Pradesh). With over 700 employees and a turnover of ₹190 million annually, the company exports to Africa, ASEAN, and Gulf countries.
Rated 3.8/5 on AmbitionBox for skill development (3.8/5), employees appreciate the learning opportunities but note challenges with work-life balance (3.5/5) due to shift work and work culture (3.2/5).
Job Opportunities at Amritsar Facility
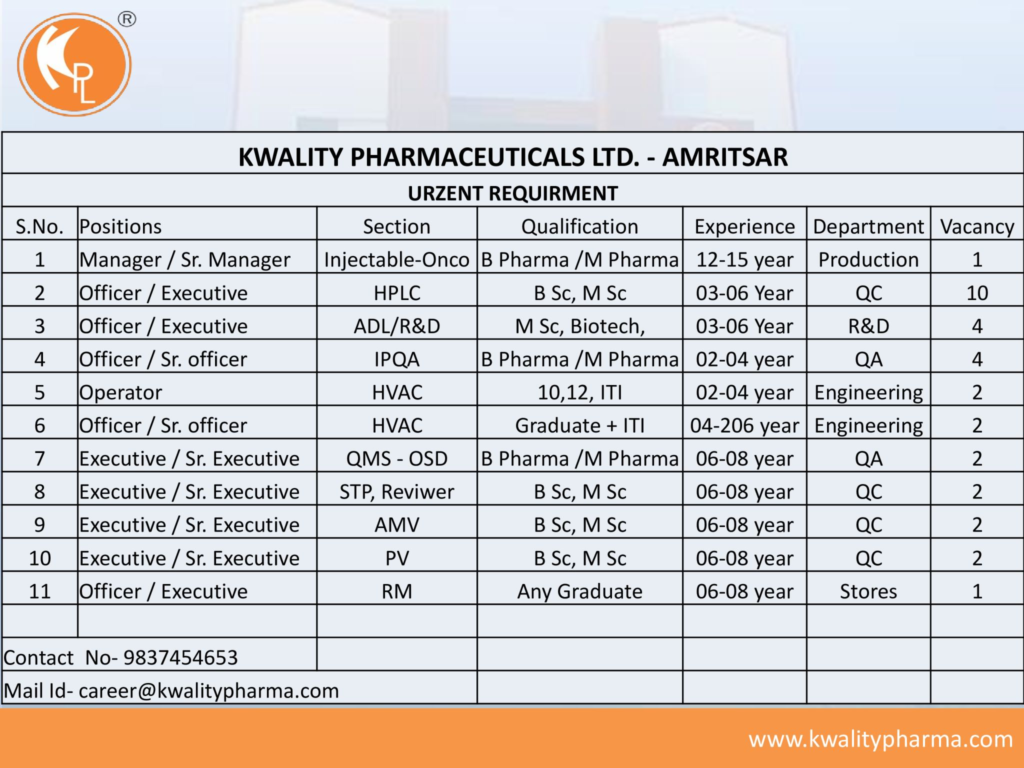
We are hiring for roles across Production, Quality Control (QC), Research & Development (R&D), Quality Assurance (QA), Engineering, and Stores departments. Candidates with experience in injectables, oncology, or OSD formulations and willingness to work rotational shifts are preferred. Below are the details.
1. Manager / Senior Manager – Injectable (Oncology)
- Qualification: B.Pharm / M.Pharm
- Experience: 12–15 years in injectable (oncology) manufacturing
- Department: Production
- Section: Injectable-Oncology
- Responsibilities:
- Oversee oncology injectable production, ensuring WHO-GMP compliance
- Manage process optimization, scale-up, and technology transfer
- Lead team training and regulatory audits (USFDA, WHO)
- Required Skills:
- Expertise in aseptic processes and oncology injectables
- Strong leadership and GMP knowledge
- Audit readiness (USFDA, WHO, EU-GMP)
- Vacancies: 1
2. Officer / Executive – HPLC
- Qualification: B.Sc / M.Sc (Chemistry)
- Experience: 3–6 years in HPLC analysis
- Department: Quality Control
- Section: HPLC
- Responsibilities:
- Perform HPLC analysis for raw materials, intermediates, and finished products
- Troubleshoot HPLC instruments and ensure GLP compliance
- Maintain accurate documentation for regulatory audits
- Required Skills:
- Proficiency in HPLC operation and troubleshooting
- Knowledge of GMP, GLP, and ICH guidelines
- Analytical and documentation skills
- Vacancies: 10
3. Officer / Executive – ADL/R&D
- Qualification: M.Sc (Biotechnology)
- Experience: 3–6 years in analytical development or R&D
- Department: Research & Development
- Section: ADL/R&D
- Responsibilities:
- Develop and validate analytical methods for new formulations
- Conduct stability studies and impurity profiling
- Support formulation development and technology transfer
- Required Skills:
- Experience in method development and validation
- Familiarity with HPLC, GC, and spectroscopy
- Knowledge of GMP and regulatory requirements
- Vacancies: 4
4. Officer / Senior Officer – IPQA
- Qualification: B.Pharm / M.Pharm
- Experience: 2–4 years in IPQA
- Department: Quality Assurance
- Section: IPQA
- Responsibilities:
- Perform in-process quality checks for manufacturing and packing
- Review BMR/BPR and ensure line clearance
- Support CAPA, deviation handling, and regulatory audits
- Required Skills:
- Knowledge of IPQA processes and GMP
- Experience with QMS and audit compliance
- Attention to detail and documentation skills
- Vacancies: 4
5. Operator – HVAC
- Qualification: 10th/12th / ITI
- Experience: 2–4 years in HVAC operations
- Department: Engineering
- Section: HVAC
- Responsibilities:
- Operate and maintain HVAC systems in cleanroom environments
- Perform preventive maintenance and troubleshoot issues
- Ensure compliance with GMP and safety standards
- Required Skills:
- Hands-on experience with HVAC systems
- Basic knowledge of GMP and cleanroom standards
- Troubleshooting and maintenance skills
- Vacancies: 2
6. Officer / Senior Officer – HVAC
- Qualification: Graduate + ITI
- Experience: 4–6 years in HVAC operations
- Department: Engineering
- Section: HVAC
- Responsibilities:
- Supervise HVAC operations and maintenance in sterile areas
- Coordinate with vendors for system calibration and validation
- Ensure GMP compliance and audit readiness
- Required Skills:
- Expertise in HVAC system management
- Knowledge of GMP, cleanroom standards, and validation
- Leadership and coordination skills
- Vacancies: 2
7. Executive / Senior Executive – QMS (OSD)
- Qualification: B.Pharm / M.Pharm
- Experience: 6–8 years in QMS for OSD
- Department: Quality Assurance
- Section: QMS-OSD
- Responsibilities:
- Manage QMS activities (CAPA, deviations, change control)
- Conduct internal audits and prepare for regulatory inspections
- Ensure compliance with WHO-GMP and QMS documentation
- Required Skills:
- In-depth knowledge of QMS and OSD processes
- Experience with regulatory audits (USFDA, WHO)
- Strong analytical and problem-solving skills
- Vacancies: 2
8. Executive / Senior Executive – STP Reviewer
- Qualification: B.Sc / M.Sc
- Experience: 6–8 years in STP review
- Department: Quality Control
- Section: STP Reviewer
- Responsibilities:
- Review Standard Testing Procedures (STPs) for accuracy
- Ensure compliance with pharmacopoeial standards
- Support QC documentation and regulatory audits
- Required Skills:
- Expertise in STP review and pharmacopoeial compliance
- Knowledge of GMP and QC documentation
- Attention to detail and regulatory knowledge
- Vacancies: 2
9. Executive / Senior Executive – AMV
- Qualification: B.Sc / M.Sc
- Experience: 6–8 years in analytical method validation
- Department: Quality Control
- Section: AMV
- Responsibilities:
- Perform analytical method validation for new and existing products
- Document validation protocols and reports
- Support regulatory submissions and audits
- Required Skills:
- Proficiency in AMV and analytical techniques (HPLC, GC)
- Knowledge of ICH guidelines and GMP
- Strong documentation and analytical skills
- Vacancies: 2
10. Executive / Senior Executive – PV
- Qualification: B.Sc / M.Sc
- Experience: 6–8 years in process validation
- Department: Quality Control
- Section: PV
- Responsibilities:
- Conduct process validation for manufacturing processes
- Prepare validation protocols and reports
- Ensure compliance with GMP and regulatory requirements
- Required Skills:
- Expertise in process validation and GMP
- Familiarity with regulatory audits and documentation
- Analytical and problem-solving skills
- Vacancies: 2
11. Officer / Executive – RM
- Qualification: Any Graduate
- Experience: 6–8 years in raw material management
- Department: Stores
- Section: RM
- Responsibilities:
- Manage raw material receipt, storage, and issuance
- Maintain inventory records and ensure GMP compliance
- Coordinate with QC for material testing and release
- Required Skills:
- Experience in raw material handling and inventory management
- Knowledge of GMP and warehouse operations
- Organizational and documentation skills
- Vacancies: 1
Application Details
- How to Apply: Share your resume via email to career@kwalitypharma.com with the subject “Application for [Position Name] – Amritsar – June 2025.” Alternatively, contact HR at +91 9837454653 for walk-in details.
- Application Deadline: Not specified; apply by June 15, 2025, for priority consideration.
- Contact: career@kwalitypharma.com | +91 9837454653
- Website: www.kwalitypharma.com
Documents Required
- Updated resume with passport-sized photograph
- Copies of educational certificates and mark sheets
- Experience certificates and relieving letters
- Last 3 months’ payslips and increment letter
- Aadhaar Card and PAN Card
Why Join Kwality Pharmaceuticals?
Kwality Pharmaceuticals offers a robust platform for skill development (3.8/5) in a WHO-GMP-certified facility with exposure to oncology injectables and OSD formulations.
Employees appreciate the learning environment and job security (3.6/5), though rotational shifts and work pressure may impact work-life balance (3.5/5). Join a company with a strong presence in regulated markets like Africa and Gulf countries
Why Amritsar?
Amritsar’s Nag Kalan facility is a key pharmaceutical hub with modern infrastructure, offering career stability. The location is accessible but may require commuting, with limited public transport noted by employees.
Important Notes
- Eligibility: Male candidates preferred for roles involving rotational shifts (Production, Engineering). Experience in injectables, oncology, or OSD required for most roles.
- Disclaimer: Kwality Pharmaceuticals does not charge fees for job applications. Use only official contact career@kwalitypharma.com or +91 9837454653. Beware of fraudulent offers.
- Note: Candidates interviewed in the last 6 months need not reapply. Expect a high-pressure environment with opportunities for technical expertise.
Don’t miss this urgent opportunity to join Kwality Pharmaceuticals Ltd.! Apply now and contribute to high-quality pharmaceutical manufacturing in Amritsar!