Gland Pharma Limited, a globally renowned manufacturer of injectables and APIs founded in 1978, is hosting a walk-in interview for API-experienced professionals in Production and Quality Control (Microbiology and Chemistry) at its facility in JN PharmaCity, Parawada, Visakhapatnam.
With a presence in 60+ countries and USFDA, EU-GMP, and WHO-GMP certifications, Gland Pharma is a leader in sterile APIs and parenterals. Join our team to advance your career in a world-class pharmaceutical environment!
Contents
About Gland Pharma Ltd.
Headquartered in Hyderabad, Gland Pharma operates multiple manufacturing facilities, including its API unit in Visakhapatnam. Known for its expertise in sterile APIs, injectables, and complex generics, the company employs over 4,000 professionals and reported a revenue of ₹5,665 crore in FY24.
Rated 3.7/5 on AmbitionBox for job security (3.9/5), employees praise learning opportunities (3.8/5) but note challenges with work-life balance (3.5/5) due to rotational shifts and high-pressure timelines. The Visakhapatnam facility is a key hub for API production, focusing on sterile processes.
Open Positions at JN PharmaCity, Visakhapatnam
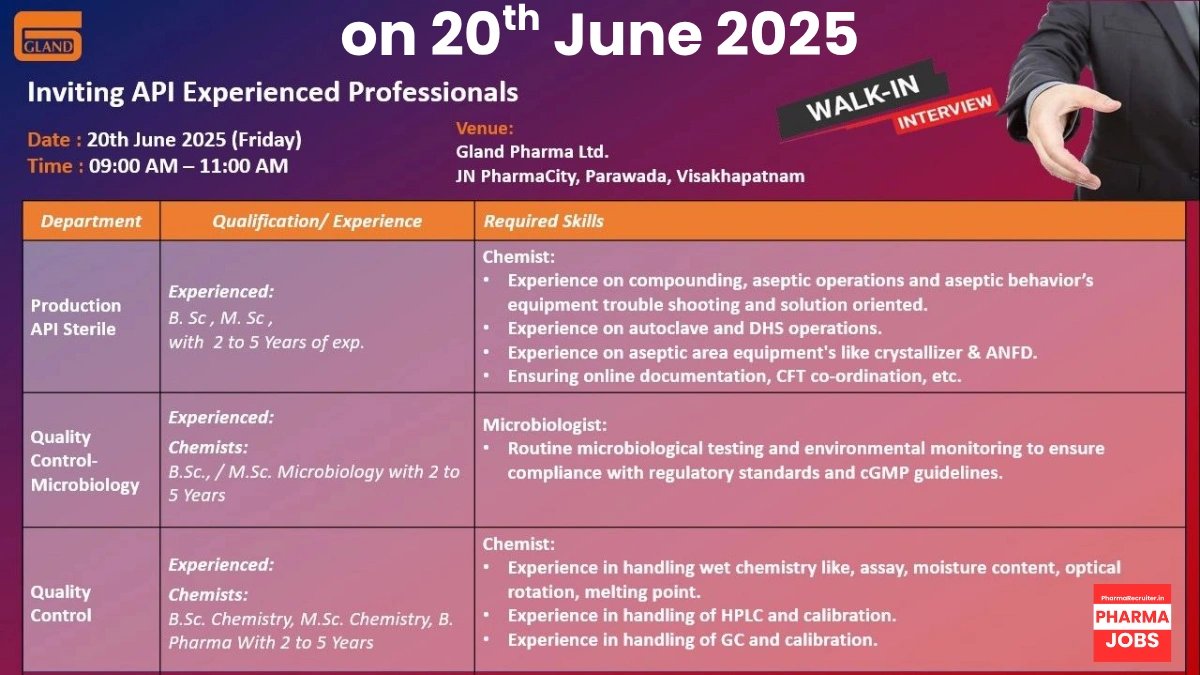
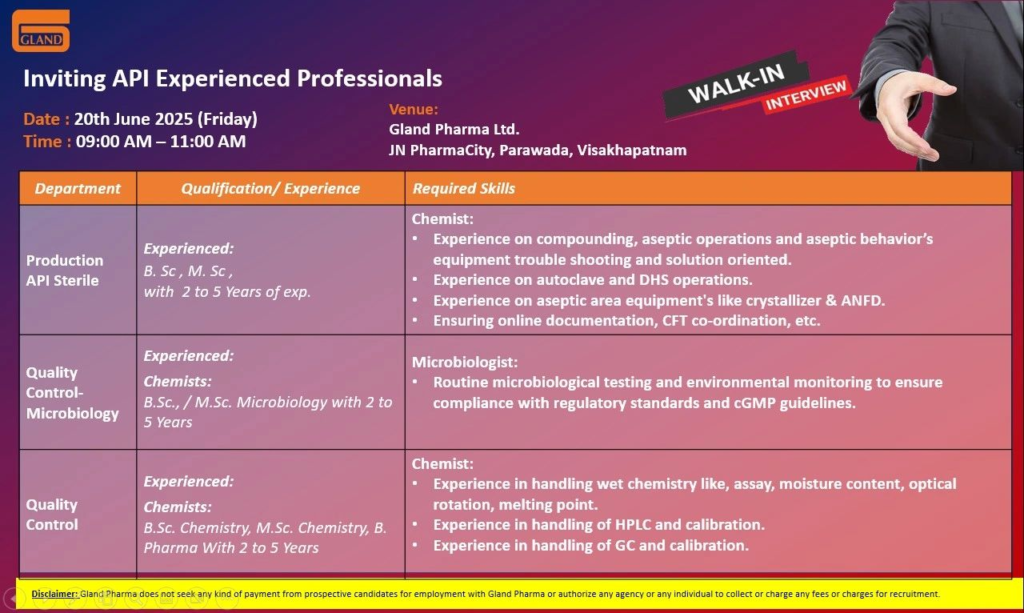
Gland Pharma is hiring Chemists and Microbiologists with 2–5 years of experience in API sterile production and quality control for its Visakhapatnam facility. Below are the details.
Production – Chemist (API Sterile)
- Qualification: B.Sc / M.Sc (Chemistry)
- Experience: 2–5 years in API sterile manufacturing
- Work Location: JN PharmaCity, Parawada, Visakhapatnam, Andhra Pradesh
- Responsibilities:
- Perform compounding and aseptic operations in sterile API production
- Operate and troubleshoot equipment like crystallizers, ANFDs, autoclaves, and DHS
- Ensure online documentation and compliance with cGMP standards
- Coordinate with cross-functional teams (CFTs) for process efficiency
- Required Skills:
- Expertise in aseptic behaviors and sterile manufacturing processes
- Hands-on experience with autoclaves, DHS, crystallizers, and ANFDs
- Strong problem-solving and documentation skills
- Knowledge of GMP and regulatory audits (USFDA, EU-GMP)
- Vacancies: Multiple
- Note: Rotational shifts mandatory; male candidates preferred due to shift work.
Quality Control (Microbiology) – Chemist / Microbiologist
- Qualification: B.Sc / M.Sc (Microbiology)
- Experience: 2–5 years in microbiology testing for API/pharma
- Work Location: JN PharmaCity, Parawada, Visakhapatnam, Andhra Pradesh
- Responsibilities:
- Conduct routine microbiological testing (e.g., sterility, BET, MLT)
- Perform environmental monitoring in cleanrooms and sterile areas
- Ensure compliance with cGMP, USFDA, and WHO guidelines
- Prepare and review microbiological reports and SOPs
- Required Skills:
- Proficiency in microbiological techniques and environmental monitoring
- Knowledge of cGMP, GLP, and regulatory standards
- Experience with cleanroom protocols and documentation
- Attention to detail and analytical skills
- Vacancies: Multiple
- Note: General shift with occasional flexibility for testing schedules.
Quality Control (Chemistry) – Chemist
- Qualification: B.Sc / M.Sc (Chemistry) / B.Pharm
- Experience: 2–5 years in API/pharma quality control
- Work Location: JN PharmaCity, Parawada, Visakhapatnam, Andhra Pradesh
- Responsibilities:
- Perform wet chemistry analysis (assay, moisture content, optical rotation, melting point)
- Conduct HPLC and GC analysis, including method validation and calibration
- Ensure compliance with ICH, USFDA, and cGMP guidelines
- Maintain accurate documentation and prepare quality reports
- Required Skills:
- Expertise in HPLC (e.g., Shimadzu Lab Solutions) and GC operations
- Proficiency in wet chemistry and instrument calibration
- Knowledge of GMP, ICH guidelines, and regulatory audits
- Strong analytical and documentation skills
- Vacancies: Multiple
- Note: General shift with occasional extended hours for analysis.
Walk-In Interview Details
- Date: 20th June 2025 (Friday)
- Time: 9:00 AM – 11:00 AM
- Venue: Gland Pharma Ltd., JN PharmaCity, Parawada, Visakhapatnam, Andhra Pradesh – 531021
- Contact: Not specified; use venue for inquiries
- Email: Not specified; bring documents to walk-in
- Website: www.glandpharma.com
Documents to Bring
- Updated resume with passport-sized photograph
- Copies of educational certificates (B.Sc/M.Sc/B.Pharm)
- Aadhaar Card and PAN Card
- Last 3 months’ payslips and latest increment letter
- Experience certificates or relieving letters
- Last 3 months’ bank statement
How to Apply
Attend the walk-in interview at Gland Pharma’s JN PharmaCity facility in Visakhapatnam with the required documents. If unable to attend, email your resume to careers@glandpharma.com with the subject “Application for [Position Name] – Visakhapatnam – June 2025” (based on standard company practice).
Only candidates with 2–5 years of API/pharma experience are eligible. Immediate joiners are preferred.
Why Join Gland Pharma?
Gland Pharma offers a platform to work in a USFDA-approved API facility with cutting-edge sterile manufacturing processes. Employees benefit from:
- Learning Opportunities: Exposure to advanced API production and QC techniques (3.8/5)
- Global Reach: Work with a company serving 60+ countries
- Job Security: Strong stability in a leading pharma firm (3.9/5)
- Benefits: Competitive salaries (avg. ₹3–5L for chemists), transport, and canteen facilities However, rotational shifts in Production and high-pressure timelines may impact work-life balance (3.5/5). Join a global leader in sterile APIs and injectablesFailed to load imageView link
Why JN PharmaCity, Visakhapatnam?
JN PharmaCity, Parawada, is a major pharmaceutical hub in Andhra Pradesh, hosting multiple API and formulation units.
Gland Pharma’s facility is accessible but may require personal transport due to limited public connectivity. Visakhapatnam offers a coastal lifestyle with moderate living costs compared to Hyderabad.
Important Notes
- Eligibility: Only candidates with 2–5 years of API/pharma experience (sterile preferred). Male candidates preferred for Production due to rotational shifts.
- Rotational Shifts: Mandatory for Production roles; QC roles are general shift.
- Disclaimer: Gland Pharma does not charge fees for job applications. Use only official channels (walk-in or careers@glandpharma.com). Beware of fraudulent offers.
- Note: Candidates interviewed in the last 6 months are not eligible. Expect a technical interview focusing on API processes, HPLC/GC, and microbiology techniques.
Don’t miss this opportunity to join Gland Pharma Ltd.! Attend our walk-in interview on 20th June 2025 in Visakhapatnam and contribute to world-class API manufacturing!