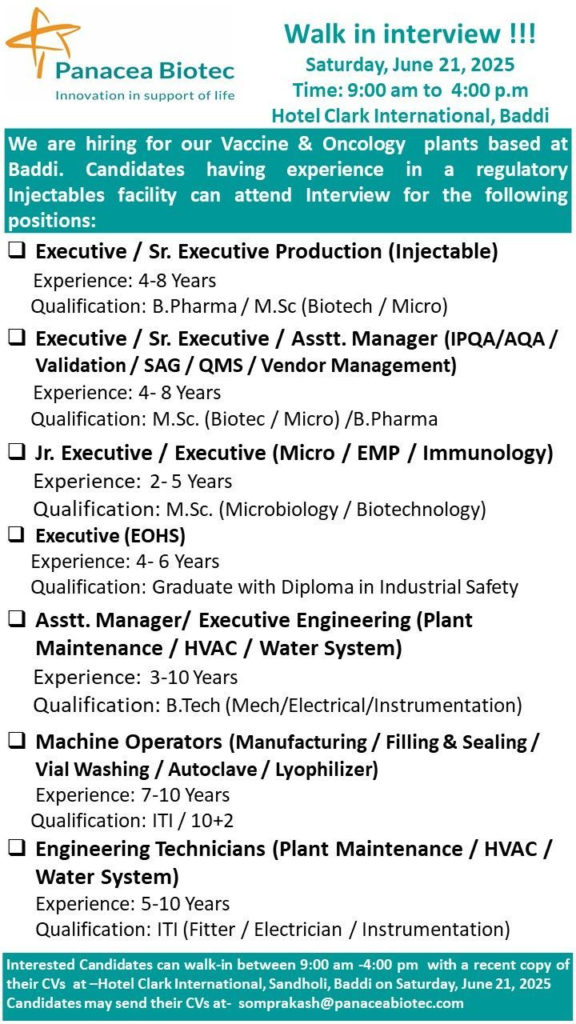
Panacea Biotec, a global leader in pharmaceuticals and biotechnology, is hosting a Walk-in Interview on June 21, 2025, in Baddi, Himachal Pradesh. We’re hiring for multiple positions at our Vaccine and Oncology plants, supporting our mission of Innovation in Support of Life.
About Panacea Biotec
Founded in 1984, Panacea Biotec is renowned for its innovative vaccines and oncology products, with a presence in over 60 countries. Our Baddi facilities are CGMP-compliant, serving global markets with high-quality injectables. Learn more at Panacea Biotec.
Walk-in Interview Details
We’re seeking experienced professionals for our Baddi Vaccine and Oncology plants. Candidates with expertise in regulatory injectables facilities are encouraged to attend.
| Event Details | Information |
|---|---|
| Date | June 21, 2025 (Saturday) |
| Time | 9:00 AM to 4:00 PM IST |
| Venue | Hotel Clark International, Sandholi, Baddi, Himachal Pradesh |
| Application Email | somprakash@panaceabiotec.com |
Job Opportunities
We are hiring for the following roles, all requiring experience in a regulatory injectables facility:
1. Executive / Sr. Executive – Production (Injectable)
- Qualification: B.Pharma / M.Sc. (Biotech / Microbiology).
- Experience: 4–8 years.
- Responsibilities:
- Oversee injectable manufacturing (vials, pre-filled syringes).
- Ensure compliance with CGMP and SOPs.
- Optimize production efficiency and quality.
- Skills: Expertise in aseptic processing, fill-finish, and regulatory audits.
2. Executive / Sr. Executive / Asst. Manager – QA (IPQA/AQA/Validation/SAG/QMS/Vendor Management)
- Qualification: M.Sc. (Biotech / Microbiology) / B.Pharma.
- Experience: 4–8 years.
- Responsibilities:
- Conduct in-process quality assurance (IPQA) and analytical QA (AQA).
- Manage validation, QMS, and vendor audits.
- Support self-assessment group (SAG) activities.
- Skills: Knowledge of QMS, CAPA, and regulatory compliance.
3. Jr. Executive / Executive – Microbiology / EMP / Immunology
- Qualification: M.Sc. (Microbiology / Biotechnology).
- Experience: 2–5 years.
- Responsibilities:
- Perform environmental monitoring (EMP) and microbiological testing.
- Support immunology assays for vaccine quality.
- Maintain compliance with GLP standards.
- Skills: Proficiency in sterility testing, bioburden, and LAL testing.
4. Executive – EOHS (Environment, Occupational Health & Safety)
- Qualification: Graduate with Diploma in Industrial Safety.
- Experience: 4–6 years.
- Responsibilities:
- Implement EOHS policies for plant safety.
- Conduct risk assessments and safety audits.
- Ensure compliance with OSHA and local regulations.
- Skills: Expertise in hazard identification and safety training.
5. Asst. Manager / Executive – Engineering (Plant Maintenance / HVAC / Water System)
- Qualification: B.Tech (Mechanical / Electrical / Instrumentation).
- Experience: 3–10 years.
- Responsibilities:
- Manage plant maintenance, HVAC, and water systems.
- Ensure equipment uptime and regulatory compliance.
- Oversee preventive maintenance schedules.
- Skills: Knowledge of HVAC validation, purified water systems, and automation.
6. Machine Operators (Manufacturing / Filling & Sealing / Vial Washing / Autoclave / Lyophilizer)
- Qualification: ITI / 10+2.
- Experience: 7–10 years.
- Responsibilities:
- Operate aseptic filling, vial washing, autoclave, or lyophilizer equipment.
- Follow SOPs for production processes.
- Ensure cleanroom compliance.
- Skills: Experience with injectable manufacturing equipment.
7. Engineering Technicians (Plant Maintenance / HVAC / Water System)
- Qualification: ITI (Fitter / Electrician / Instrumentation).
- Experience: 5–10 years.
- Responsibilities:
- Support maintenance of plant equipment, HVAC, and water systems.
- Perform troubleshooting and repairs.
- Assist in equipment qualification.
- Skills: Proficiency in mechanical, electrical, or instrumentation maintenance.
Eligibility Criteria
- Qualifications:
- B.Pharma, M.Sc. (Biotech/Microbiology), B.Tech (Mech/Elect/Instr), Graduate with Industrial Safety Diploma, ITI, or 10+2.
- Experience: 2–10 years in a regulatory injectables facility (vaccine/oncology preferred).
- Skills:
- Familiarity with CGMP, GLP, and regulatory audits (USFDA, EMA).
- Expertise in aseptic processing, QMS, or equipment maintenance.
- Strong problem-solving and teamwork abilities.
Why Join Panacea Biotec?
Panacea Biotec offers a rewarding career in a globally recognized organization, known for vaccines like EasySix and oncology injectables. Benefits include:
- Competitive Salaries: Sr. Executive roles average ₹6-10 LPA in Baddi.
- Global Exposure: Work in USFDA/EMA-approved facilities.
- Career Growth: Training in QMS, validation, and bioprocessing.
- Innovative Culture: Contribute to novel vaccine and oncology products.
- Stable Environment: Rated 3.5/5 for job security by employees.
Why These Roles Matter
These positions are vital to Panacea’s Baddi plants, producing vaccines and oncology injectables for global markets. From ensuring aseptic production to maintaining quality compliance, your work will impact healthcare worldwide. With 1,200 pharmaceutical jobs in Baddi, Panacea stands out for its regulatory excellence.
Growth Opportunities
Panacea invests in training for regulatory compliance, equipment handling, and safety standards. The Baddi facility, a hub for 2,500 professionals, offers exposure to advanced manufacturing and QA processes. Employees value learning opportunities but note a demanding work pace.
Work Environment
Our Baddi team thrives in a fast-paced, quality-driven environment. We prioritize precision, innovation, and collaboration, ensuring access to modern cleanrooms and labs. Panacea’s focus on employee safety and compliance creates a supportive workplace.
How to Attend
Join us on June 21, 2025, from 9:00 AM to 4:00 PM at Hotel Clark International, Sandholi, Baddi. Bring:
- Recent CV.
- Educational certificates.
- Experience letters or payslips.
- Photo ID and passport-size photos.
Alternatively, email your CV to somprakash@panaceabiotec.com with the position title in the subject line. Immediate joiners preferred.
Preparation Tips
- Highlight injectables experience in vaccines or oncology.
- Showcase expertise in CGMP, QMS, or equipment maintenance.
- Bring examples of audit compliance or process optimization.
- Ensure documents are ready for quick verification.
Important Disclaimer
Panacea Biotec maintains a transparent recruitment process. We do not charge fees or use free email services (e.g., Gmail, Yahoo) for job offers. Verify opportunities through somprakash@panaceabiotec.com or Panacea Biotec Careers. Report suspicious activities via our contact page.
Stay Safe from Fraud
- Confirm offers through official Panacea channels.
- Avoid sharing personal or financial information with unverified sources.
- Contact HR for recruitment clarifications.
Why Baddi?
Baddi, Himachal Pradesh, is India’s pharmaceutical hub, hosting over 700 CGMP-compliant plants. Panacea’s facilities offer a modern workspace with scenic surroundings, ideal for professionals seeking growth in a thriving industry.
Join Panacea’s Mission
Panacea Biotec is dedicated to Innovation in Support of Life. By joining our Baddi team, you’ll contribute to life-saving vaccines and oncology therapies. Attend our walk-in interview or email your CV to start your journey.
Next Steps
Arrive early for registration on June 21, 2025. The selection process may include technical interviews and assessments. Selected candidates will receive spot offers post-verification. Check emails for updates from somprakash@panaceabiotec.com.
Contact Us
For queries, email somprakash@panaceabiotec.com or visit our contact page. We’re here to support your application.
Advance Your Career
Join Panacea Biotec to drive innovation in vaccines and oncology. Attend our walk-in interview on June 21, 2025, in Baddi or email your CV today!


