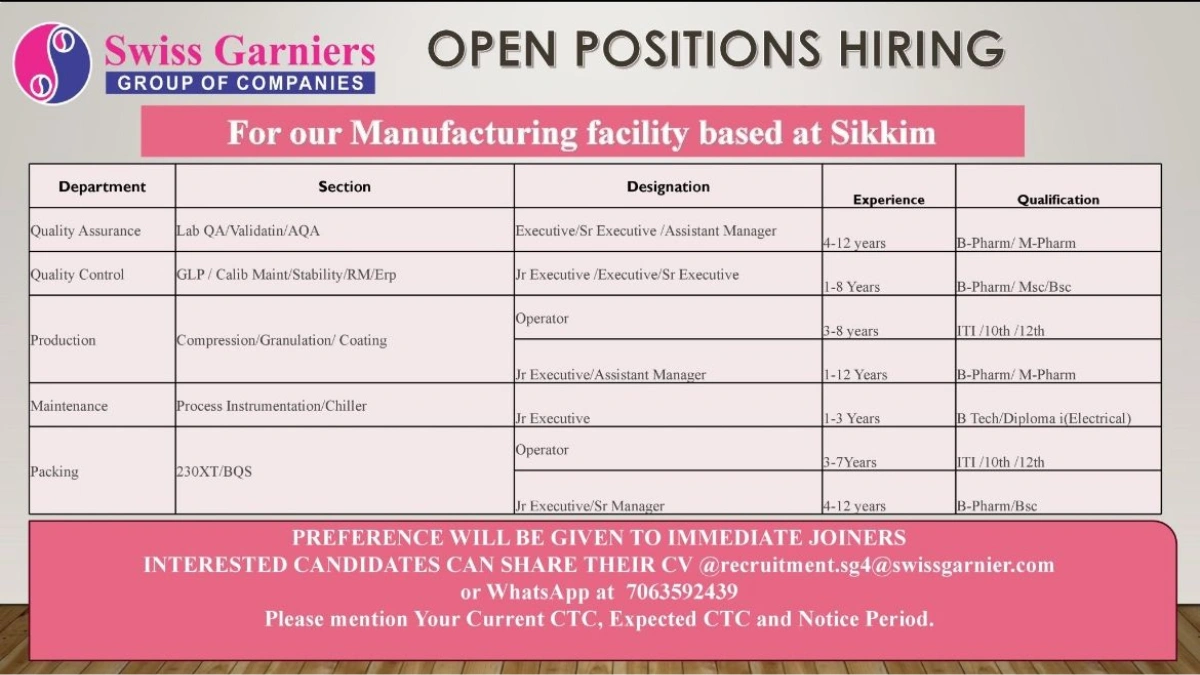
Swiss Garnier, a global pharmaceutical manufacturer specializing in innovative formulations, is hiring for its state-of-the-art manufacturing facility in Sikkim, India. Part of a dynamic group of companies, we are seeking skilled professionals to join our team in Quality Assurance, Quality Control, Production, Maintenance, and Packing departments.
Our USFDA and PIC/s-approved facility in Mamring, Sikkim, spans 273,000 sq. ft. on 7.30 acres, focusing on tablets, capsules, liquids, ointments, and powders for 40+ countries.
Contents
About Swiss Garnier
Incorporated in 2006, Swiss Garnier has grown to a turnover of over ₹450 crore, with four manufacturing units catering to domestic and international markets. Located along the Teesta River, our Sikkim plant emphasizes quality through Six Sigma and TQM principles, employing 200+ professionals. Rated 3.6/5 on AmbitionBox for work-life balance, we prioritize innovation and compliance.
Job Opportunities
We’re hiring for multiple roles at our Sikkim manufacturing unit. Below are the open positions, qualifications, experience, and preferences:
1. Quality Assurance
- Section: Lab QA / Validation / AQA
- Designation: Executive / Sr. Executive / Assistant Manager
- Experience: 4–12 years
- Qualification: B.Pharm / M.Pharm
- Responsibilities:
- Conduct Lab QA tests and validation activities for pharmaceutical formulations.
- Ensure AQA (Analytical Quality Assurance) compliance with cGMP and USFDA standards.
- Manage deviations, CAPA, and audit preparedness.
- Skills: Expertise in validation protocols, quality systems, and regulatory compliance.
2. Quality Control
- Section: GLP / Calibration Maintenance / Stability / RM / ERP
- Designation: Jr. Executive / Executive / Sr. Executive
- Experience: 1–8 years
- Qualification: B.Pharm / M.Sc. / B.Sc.
- Responsibilities:
- Perform GLP-compliant testing and calibration maintenance of instruments.
- Monitor stability studies and analyze raw materials (RM) using HPLC or GC.
- Manage ERP systems for quality data.
- Skills: Proficiency in analytical techniques, stability testing, and documentation.
3. Production
- Section: Compression / Granulation / Coating
- Designation: Operator
- Experience: 3–8 years
- Qualification: ITI / 10th / 12th
- Responsibilities:
- Operate compression, granulation, and coating machines (e.g., Cadmach, FBD).
- Ensure batch record compliance and cleanroom protocols.
- Skills: Hands-on experience with production equipment and cGMP.
- Designation: Jr. Executive / Assistant Manager
- Experience: 1–12 years
- Qualification: B.Pharm / M.Pharm
- Responsibilities:
- Supervise production processes and optimize granulation/coating operations.
- Ensure quality control and scale-up readiness.
- Skills: Leadership in formulation production and SOP adherence.
4. Maintenance
- Section: Process Instrumentation / Chiller
- Designation: Jr. Executive
- Experience: 1–3 years
- Qualification: B.Tech / Diploma (Electrical)
- Responsibilities:
- Maintain process instrumentation and chiller systems for manufacturing.
- Perform preventive maintenance and troubleshoot issues.
- Skills: Knowledge of electrical systems and GMP-compliant maintenance.
- Designation: Operator
- Experience: 3–7 years
- Qualification: ITI / 10th / 12th
- Responsibilities:
- Support instrumentation and chiller operations in production areas.
- Skills: Basic technical skills and equipment handling.
5. Packing
- Section: 230XT / BQS
- Designation: Jr. Executive / Sr. Manager
- Experience: 4–12 years
- Qualification: B.Pharm / B.Sc.
- Responsibilities:
- Oversee packing operations using 230XT and BQS machines.
- Ensure secondary packing compliance and serialization.
- Skills: Expertise in packing line management and cGMP.
Why Join Swiss Garnier?
Swiss Garnier offers a rewarding career in a globally recognized pharmaceutical company. Benefits include:
- Competitive Salary: Executives earn ₹4–12 LPA; Managers ₹10–20 LPA in Sikkim.
- Global Exposure: Work in a USFDA/PIC/s-approved facility serving 40+ countries.
- Career Growth: Training in HPLC, validation, production techniques, and maintenance.
- Supportive Environment: Rated 3.6/5 for work-life balance and 3.2/5 for career growth (AmbitionBox).
- Challenges: Employees note high-pressure timelines and 6-day work weeks (70% report).
Why These Roles Matter
These roles drive Swiss Garnier’s production of diverse formulations, ensuring quality for global markets. Your work in QA, QC, or Production supports Sikkim’s 800+ pharma job ecosystem, contributing to innovative healthcare solutions.
Growth Opportunities
Training includes QbD, analytical methods, and GMP compliance, with exposure to global audits. Employees value learning opportunities (3.6/5) but note limited promotions (3.2/5).
Work Environment
The Sikkim facility features cleanrooms, HPLC labs, and packing lines, fostering a quality-driven culture (3.6/5 work-life balance). Expect shift-based roles with a scenic riverside location.
How to Apply
Interested candidates can apply by June 20, 2025, 5:21 PM IST, as positions are open for immediate joiners. Submit:
- Updated CV with current CTC, expected CTC, and notice period.
- Educational certificates and experience letters.
Options:
- Email: recruitment.sg4@swissgarnier.com
- WhatsApp: +91 7063592439

Preference: Immediate joiners will be prioritized. Shortlisted candidates will be contacted for interviews, which may include technical assessments (e.g., HPLC testing, machine operation).
Application Tips
- Highlight relevant experience in QA validation, QC stability, or production equipment.
- Specify immediate joiner status and provide clear CTC details.
- Prepare for questions like “How do you ensure cGMP in packing?” or “What are key maintenance checks for chillers?”
Important Disclaimer
Swiss Garnier maintains a transparent recruitment process. We do not charge fees or use free email services (e.g., Gmail, Yahoo) for job offers. Verify opportunities through recruitment.sg4@swissgarnier.com or WhatsApp +91 7063592439. Report suspicious activities to HR.
Stay Safe from Fraud
- Confirm offers through official Swiss Garnier channels.
- Avoid sharing personal or financial information with unverified sources.
- Contact HR for clarifications.
Why Sikkim?
Sikkim is an emerging pharma hub with 800+ jobs, hosting Swiss Garnier’s advanced facility. Its scenic location and tax benefits make it ideal for industrial careers.
Join Swiss Garnier’s Mission
Swiss Garnier is dedicated to delivering innovative pharmaceutical formulations globally. Join our Sikkim team to contribute to quality production. Apply now to be part of our 200+ strong workforce!
Next Steps
Submit your CV via email or WhatsApp by June 20, 2025, 5:21 PM IST. The selection process may include technical interviews and document verification. Successful candidates will receive further communication.
Contact Us
For queries, email recruitment.sg4@swissgarnier.com or WhatsApp +91 7063592439. Visit Swiss Garnier Careers for updates.
Innovate with Swiss Garnier
Join Swiss Garnier to advance pharmaceutical manufacturing in Sikkim. Apply today and drive innovation in healthcare!