KLM Laboratories Pvt. Ltd., a leading pharmaceutical company established in 2006, is hosting a Walk-in Drive on June 22, 2025, at its manufacturing unit near Vadodara, Gujarat. With a mission to improve lives through innovative healthcare solutions, KLM specializes in dermatological and pediatric products, serving markets across India and internationally with cGMP-compliant facilities. Join our team of over 1,000 professionals to contribute to quality healthcare.
Contents
About KLM Laboratories
Founded as a dermaceutical company, KLM has grown into a trusted name in pharmaceuticals, launching its pediatric division in 2016. The Vadodara plant, located at 261, GIDC Makarpura, adheres to top industry standards, including WHO-GMP and EU certifications, with a focus on semi-solid and oral dosage forms.
Walk-in Drive Details
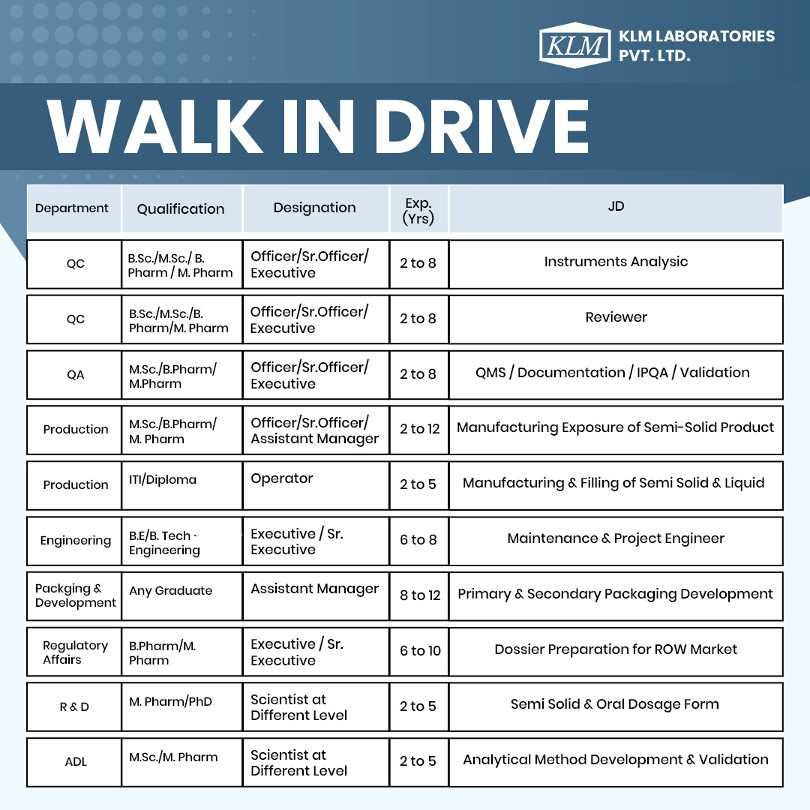
We’re hiring for roles in Quality Control, Quality Assurance, Production, Engineering, Packaging & Development, Regulatory Affairs, R&D, and ADL at our Vadodara facility.
| Event Details | Information |
|---|---|
| Date | June 22, 2025 (Sunday) |
| Time | 10:00 AM to 3:00 PM IST |
| Venue | 261, GIDC Makarpura, Bharti House, Bank of Baroda Lane, Vadodara, Gujarat – 390010 |
| Contact | +91 92278 97864 or career@klmlab.com |
| Note | Bring updated resume, CTC breakup, and photograph; only relevant experience candidates are eligible |
Job Opportunities
1. Quality Control (QC)
- Qualification: B.Sc. / M.Sc. / B.Pharm / M.Pharm
- Designation: Officer / Sr. Officer / Executive
- Experience: 2–8 years
- Role: Instruments Analysis
- Responsibilities:
- Operate and maintain analytical instruments (e.g., HPLC, GC).
- Perform testing of raw materials, in-process, and finished products.
- Ensure compliance with GLP and cGMP standards.
- Designation: Officer / Sr. Officer / Executive
- Experience: 2–8 years
- Role: Reviewer
- Responsibilities:
- Review analytical data, batch records, and validation reports.
- Ensure accuracy and regulatory compliance of QC documentation.
- Support audit preparedness.
2. Quality Assurance (QA)
- Qualification: M.Sc. / B.Pharm / M.Pharm
- Designation: Officer / Sr. Officer / Executive
- Experience: 2–8 years
- Responsibilities:
- Manage QMS, documentation, IPQA, and validation processes.
- Conduct internal audits and ensure cGMP compliance.
- Support regulatory inspections and training.
3. Production
- Qualification: M.Sc. / B.Pharm / M.Pharm
- Designation: Officer / Sr. Officer / Assistant Manager
- Experience: 2–12 years
- Role: QMS / Documentation / IPQA / Validation
- Responsibilities:
- Oversee manufacturing processes for semi-solid products.
- Ensure batch record compliance and process validation.
- Lead team training and documentation.
- Qualification: ITI / Diploma
- Designation: Operator
- Experience: 2–5 years
- Role: Manufacturing & Filling of Semi-Solid & Liquid
- Responsibilities:
- Operate filling and manufacturing equipment for semi-solid and liquid products.
- Perform cleaning and line clearance per SOPs.
- Maintain production logs.
4. Engineering
- Qualification: B.E. / B.Tech (Engineering)
- Designation: Executive / Sr. Executive
- Experience: 6–8 years
- Role: Maintenance & Project Engineer
- Responsibilities:
- Manage maintenance of production equipment.
- Lead engineering projects and ensure uptime.
- Ensure compliance with safety and GMP standards.
5. Packaging & Development
- Qualification: Any Graduate
- Designation: Assistant Manager
- Experience: 8–12 years
- Role: Primary & Secondary Packaging Development
- Responsibilities:
- Develop and optimize primary and secondary packaging solutions.
- Ensure packaging integrity and regulatory compliance.
- Lead packaging process improvements.
6. Regulatory Affairs
- Qualification: B.Pharm / M.Pharm
- Designation: Executive / Sr. Executive
- Experience: 6–10 years
- Role: Dossier Preparation for ROW Market
- Responsibilities:
- Prepare dossiers for Rest of World (ROW) markets.
- Ensure compliance with international regulatory guidelines.
- Handle submissions and query responses.
7. Research & Development (R&D)
- Qualification: M.Pharm / PhD
- Designation: Scientist at Different Levels
- Experience: 2–5 years
- Role: Semi-Solid & Oral Dosage Form
- Responsibilities:
- Develop formulations for semi-solid and oral dosage products.
- Conduct stability and efficacy studies.
- Innovate new product development.
8. Analytical Development Laboratory (ADL)
- Qualification: M.Sc. / M.Pharm
- Designation: Scientist at Different Levels
- Experience: 2–5 years
- Role: Analytical Method Development & Validation
- Responsibilities:
- Develop and validate analytical methods for new products.
- Perform method transfer and stability testing.
- Ensure regulatory compliance of analytical data.
Why Join KLM Laboratories?
KLM offers a rewarding career in a globally recognized pharmaceutical company. Benefits include:
- Competitive Salary: Officers earn ₹4–10 LPA; Managers/Scientists ₹10–20 LPA in Vadodara.
- Global Exposure: Work in a cGMP-compliant facility serving India and international markets.
- Career Growth: Training in analytical techniques, manufacturing, and regulatory affairs.
- Supportive Environment: Known for a collaborative culture with growth opportunities.
- Challenges: Employees may face high-pressure timelines and shift-based work.
Why These Roles Matter
These positions drive KLM’s production of dermatological and pediatric products, supporting Vadodara’s pharma ecosystem with over 1,500 jobs. Your role will enhance quality healthcare solutions globally.
Growth Opportunities
Training includes cGMP, method validation, and leadership skills, with exposure to international audits. Employees value skill development but note moderate career progression.
Work Environment
The Vadodara facility features advanced labs and production units, fostering a quality-driven culture. Expect shift-based roles with a structured schedule.
How to Attend
Join us on June 22, 2025, from 10:00 AM to 3:00 PM at 261, GIDC Makarpura, Vadodara. Bring:
- Updated resume.
- CTC breakup.
- Passport-size photograph.
Verified by Trusted HRs
The post is released by the KLM Laboratories LinkedIn page. Click here to visit the post

For queries, contact +91 92278 97864 or email career@klmlab.com. Note: Only candidates with relevant experience are eligible. Arrive early for registration.
Preparation Tips
- Highlight experience in QC instruments, semi-solid manufacturing, or regulatory dossiers.
- Specify years of experience matching the job criteria.
- Prepare for questions like “How do you validate an analytical method?” or “What steps ensure cGMP compliance?”
Important Disclaimer
KLM Laboratories maintains a transparent recruitment process. We do not charge fees or use free email services (e.g., Gmail, Yahoo) for job offers. Verify opportunities through +91 92278 97864 or career@klmlab.com. Report suspicious activities to HR.
Stay Safe from Fraud
- Confirm offers through official KLM channels.
- Avoid sharing personal or financial information with unverified sources.
- Contact HR for clarifications.
Why Vadodara?
Vadodara, Gujarat, is a thriving pharma hub with over 1,500 jobs, hosting KLM’s advanced manufacturing unit. Its industrial growth and connectivity to Ahmedabad (100 km) make it ideal for pharma careers.
Join KLM’s Mission
KLM Laboratories is dedicated to improving lives through innovative healthcare. Join our Vadodara team to contribute to this mission. Attend our walk-in drive on June 22, 2025, to join our growing workforce!
Next Steps
Arrive by 10:00 AM on June 22, 2025. The selection process may include technical interviews and document verification. Successful candidates will receive further communication.
Contact Us
For queries, call +91 92278 97864 or email career@klmlab.com. Visit KLM Careers for updates.
Innovate with KLM
Join KLM Laboratories to advance pharmaceutical manufacturing in Vadodara. Apply today and drive innovation in healthcare!


