Ipca Laboratories Limited, a leading Indian pharmaceutical company with a 75-year legacy, is hosting a Walk-in Interview on June 29, 2025, at its Formulation (OSD) Unit in Piparia, Silvassa.
Renowned for its wide range of APIs, formulations, and healthcare products, Ipca serves global markets, including the US and Europe, with USFDA and WHO-GMP-compliant facilities. Join our team of over 15,000 professionals to contribute to quality healthcare solutions.
Contents
- 1 Company Background
- 2 Event Details
- 3 Job Opportunities
- 4 Benefits
- 5 Why These Roles Matter
- 6 Growth Opportunities
- 7 Work Environment
- 8 How to Attend
- 9 Important Disclaimer
- 10 Safety from Fraud
- 11 Why Piparia, Silvassa?
- 12 Join Ipca’s Mission
- 13 Next Steps
- 14 Contact Us
- 15 Innovate with Ipca
Company Background
- Headquarters: Mumbai, India
- Established: 1949
- Revenue: ₹6,000 crore (FY24)
- Facilities: Advanced manufacturing units, including Piparia (Silvassa)
- Rating: 3.7/5 for work culture (AmbitionBox)
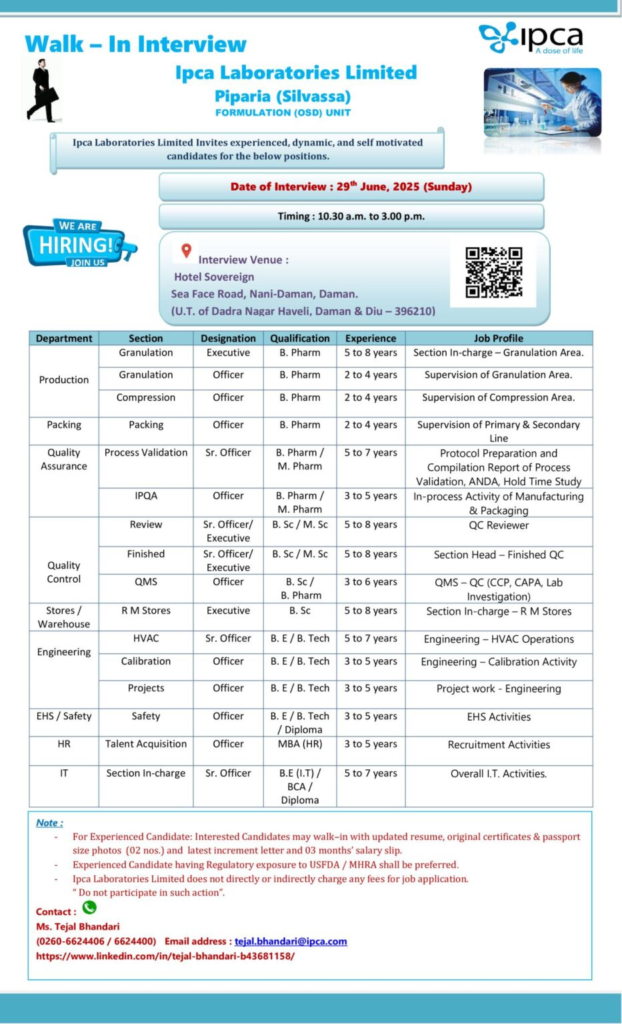
Event Details
| Field | Information |
|---|---|
| Date | June 29, 2025 (Sunday) |
| Time | 10:30 AM to 3:00 PM IST |
| Venue | Hotel Sovereign, Sea Face Road, Nani-Daman, Daman, U.T. of Dadra Nagar Haveli, Daman & Diu – 396210 |
| Contact | +91 260-6624406 / 6624400 or tejal.bhandari@ipca.com |
| Note | Bring updated resume, original certificates, 2 passport-size photos, latest increment letter, and 3 months’ salary slips; regulatory exposure to USFDA/MHRA preferred |
Job Opportunities
1. Production
Granulation
Designation: Executive
- Qualification: B.Pharm
- Experience: 5–8 years
- Job Profile: Section In-charge – Granulation Area
- Responsibilities:
- Oversee granulation processes.
- Ensure cGMP compliance.
- Manage team performance.
Designation: Officer
- Experience: 2–4 years
- Job Profile: Supervision of Granulation Area
- Responsibilities:
- Monitor granulation operations.
- Maintain batch records.
- Support process optimization.
Compression
- Designation: Officer
- Qualification: B.Pharm
- Experience: 2–4 years
- Job Profile: Supervision of Compression Area
Responsibilities:
- Supervise compression machines.
- Ensure quality checks.
- Document production.
Packing
- Designation: Officer
- Qualification: B.Pharm
- Experience: 2–4 years
- Job Profile: Supervision of Primary & Secondary Line
Responsibilities:
- Oversee packing lines.
- Ensure compliance.
- Manage line clearance.
2. Quality Assurance
Process Validation
Designation: Sr. Officer
- Qualification: B.Pharm / M.Pharm
- Experience: 5–7 years
- Job Profile: IPQA
Responsibilities:
- Conduct in-process quality checks.
- Prepare validation protocols.
- Compile reports.
Designation: Officer
- Experience: 3–5 years
- Job Profile: Protocol Preparation and Compilation Report
Responsibilities:
- Develop protocols for process validation, ANDA, and hold time studies.
- Monitor in-process activities.
Review
- Designation: Sr. Officer / Executive
- Qualification: B.Sc. / M.Sc.
- Experience: 5–8 years
- Job Profile: QC Reviewer
Responsibilities:
- Review analytical data and batch records.
- Ensure regulatory compliance.
3. Quality Control
Finished
- Designation: Sr. Officer / Executive Officer
- Qualification: B.Sc. / M.Sc.
- Experience: 5–8 years
- Job Profile: Section Head – Finished QC
Responsibilities:
- Lead finished product testing.
- Oversee lab operations.
- Ensure GLP compliance.
QMS
- Qualification: B.Sc. / B.Pharm
- Experience: 3–6 years
- Job Profile: QMS – QC (CAPA, Lab Investigation)
Responsibilities:
- Manage corrective actions.
- Investigate lab deviations.
- Ensure quality systems.
4. Stores/Warehouse
RM Stores
- Designation: Executive
- Qualification: B.Sc.
- Experience: 5–8 years
- Job Profile: Section In-charge – RM Stores
Responsibilities:
- Manage raw material inventory.
- Ensure proper storage.
- Coordinate with production.
5. Engineering
HVAC
- Designation: Sr. Officer
- Qualification: B.E. / B.Tech
- Experience: 5–7 years
- Job Profile: Engineering – HVAC Operations
Responsibilities:
- Maintain HVAC systems.
- Ensure cGMP compliance.
- Support validations.
Calibration
- Designation: Officer
- Qualification: B.E. / B.Tech
- Experience: 3–5 years
- Job Profile: Engineering Calibration Activity
Responsibilities:
- Perform calibration of instruments.
- Ensure accuracy per SOPs.
Projects
- Designation: Officer
- Qualification: B.E. / B.Tech
- Experience: 3–5 years
- Job Profile: Project Work Engineering
Responsibilities:
- Assist in engineering projects.
- Handle installation and commissioning.
6. EHS/Safety
- Section: Safety
- Designation: Officer
- Qualification: B.E. / B.Tech / Diploma
- Experience: 3–5 years
- Job Profile: EHS Activities
Responsibilities:
- Implement safety protocols.
- Conduct risk assessments.
- Ensure compliance.
7. HR
Talent Acquisition
- Designation: Officer
- Qualification: MBA (HR)
- Experience: 3–5 years
- Job Profile: Recruitment Activities
Responsibilities:
- Manage end-to-end recruitment.
- Handle screening and onboarding.
IT
- Designation: Sr. Officer
- Qualification: B.E (I.T.) / BCA / Diploma
- Experience: 5–7 years
- Job Profile: Overall IT Activities
Responsibilities:
- Oversee IT operations.
- Ensure system maintenance and data integrity.
Benefits
- Competitive Salary: Officers earn ₹4–10 LPA; Executives/Sr. Officers ₹8–15 LPA in Silvassa.
- Global Exposure: USFDA/WHO-GMP-approved facility serving international markets.
- Career Growth: Training in cGMP, validation, and leadership skills.
- Work Culture: Rated 3.7/5 (AmbitionBox).
- Challenges: High-pressure timelines and shift-based work.
Why These Roles Matter
These positions support Ipca’s OSD formulation production, contributing to Silvassa’s pharma ecosystem with over 1,000 jobs. Your role ensures quality and compliance for global healthcare.
Growth Opportunities
- Training in cGMP, analytical techniques, and project management.
- Exposure to USFDA/MHRA audits.
- Potential for advancement into leadership roles.
Work Environment
- Modern production and lab setups.
- Quality-driven culture.
- Shift-based roles with structured schedules.
How to Attend
- Date/Time: June 29, 2025, 10:30 AM–3:00 PM
- Location: Hotel Sovereign, Nani-Daman, Daman
- Requirements: Updated resume, original certificates, 2 passport-size photos, increment letter, 3 months’ salary slips
- Contact: Ms. Tejal Bhandari (+91 260-6624406 / 6624400, tejal.bhandari@ipca.com)
Preparation Tips:
- Highlight experience in relevant sections (e.g., granulation, QA validation).
- Specify USFDA/MHRA exposure if applicable.
- Prepare for questions like “How do you ensure cGMP compliance?” or “What steps do you take for process validation?”
Important Disclaimer
- Ipca does not charge fees for job applications.
- Avoid fraudulent schemes; report to HR.
- Verify via +91 260-6624406 or tejal.bhandari@ipca.com.
Safety from Fraud
- Confirm offers through official Ipca channels.
- Avoid sharing personal/financial details with unverified sources.
- Contact Tejal Bhandari for clarifications.
Why Piparia, Silvassa?
- Part of a pharma hub with over 1,000 jobs.
- Tax-free status and proximity to Mumbai (150 km).
- Ideal for pharma careers.
Join Ipca’s Mission
Ipca is committed to delivering quality pharmaceuticals globally. Attend our walk-in on June 29, 2025, to join our team!
Next Steps
- Arrive by 10:30 AM on June 29, 2025.
- Selection process: Technical interviews and document verification.
- Successful candidates will receive further communication.
Contact Us
- Phone: +91 260-6624406 / 6624400
- Email: tejal.bhandari@ipca.com
- LinkedIn: Tejal Bhandari
- Website: Ipca Careers
Innovate with Ipca
Join Ipca Laboratories to advance pharmaceutical manufacturing in Silvassa. Apply today and drive innovation in healthcare!


