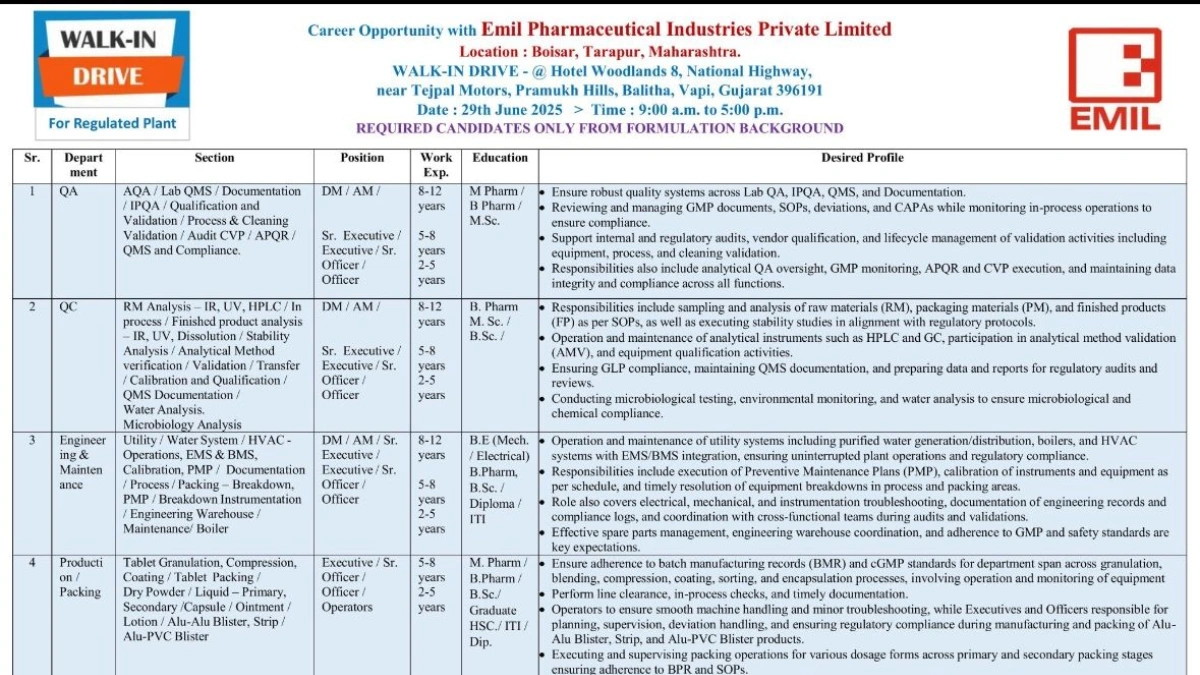
Emil Pharmaceutical Industries Private Limited, a leading name in formulation manufacturing, is hosting a Walk-In Drive for talented professionals with a formulation background on June 29, 2025, at Hotel Woodlands, Vapi, Gujarat. Join us to explore roles across multiple departments and contribute to our commitment to quality and innovation in drug development.
Contents
Event Details
- Date: June 29, 2025
- Time: 9:00 AM to 5:30 PM
- Location: Hotel Woodlands, 8, National Highway, Near Tejpal Motors, Pramukh Hills, Balitha, Vapi, Gujarat 396847
- Eligibility: Candidates with experience in formulation manufacturing only
Open Positions and Responsibilities
We are seeking skilled professionals for roles in Quality Assurance (QA), Quality Control (QC), Maintenance & Engineering, Production, and Warehouse. Below is a detailed overview of the departments, roles, qualifications, and responsibilities.
1. Quality Assurance (QA)
- Sections: AQA, Lab QA, QMS, Documentation, IPQA, Qualification & Validation, Process & Cleaning Validation, Audit, CVP, APQR, Compliance
- Positions: Deputy Manager (DM), Assistant Manager (AM), Senior Executive, Executive, Senior Officer, Officer
- Experience: 2-12 years
- Education: B.Pharm, M.Pharm, M.Sc.
Responsibilities:
- Ensure robust quality systems for Lab QA, IPQA, QMS, and documentation.
- Review and manage GMP documents, SOPs, deviations, and CAPAs.
- Monitor in-process operations to ensure compliance with regulatory standards.
- Support internal and regulatory audits, vendor qualifications, and lifecycle management of validation activities.
- Oversee analytical QA, APQR, CVP execution, and maintain data integrity.
2. Quality Control (QC)
- Sections: RM Analysis, In-Process, Finished Product Analysis, Stability, QMS Documentation, Water Analysis, Microbiology, Analytical Method Validation/Transfer
- Positions: DM, AM, Senior Executive, Executive, Senior Officer, Officer
- Experience: 2-12 years
- Education: B.Pharm, M.Sc., B.Sc.
Responsibilities:
- Conduct sampling and analysis of raw materials (RM), packaging materials (PM), and finished products (FP) using IR, UV, HPLC, and GC.
- Execute stability studies and analytical method validation (AMV).
- Operate and maintain analytical instruments, ensuring GLP compliance.
- Perform microbiological testing, environmental monitoring, and water analysis.
- Prepare data and reports for regulatory audits.
3. Maintenance & Engineering
- Sections: Utility, Water System, HVAC, EMS & BMS, Calibration, PMP, Process/Packing Breakdown, Instrumentation, Boiler
- Positions: DM, AM, Senior Executive, Executive, Senior Officer, Officer
- Experience: 2-12 years
- Education: B.E. (Mechanical/Electrical), B.Pharm, B.Sc., Diploma, ITI
Responsibilities:
- Operate and maintain utility systems (purified water, boilers, HVAC) with EMS/BMS integration.
- Execute preventive maintenance plans (PMP) and resolve equipment breakdowns.
- Perform calibration of instruments and troubleshoot electrical, mechanical, and instrumentation issues.
- Manage spare parts, engineering warehouse, and GMP/safety compliance documentation.
4. Production & Packing
- Sections: Tablet Granulation, Compression, Coating, Dry Powder, Liquid, Capsule, Ointment, Lotion, Alu-Alu Blister, Strip, Alu-PVC Blister
- Positions: Executive, Senior Officer, Officer, Operators
- Experience: 2-8 years
- Education: M.Pharm, B.Pharm, B.Sc., Graduate, HSC, ITI, Diploma
Responsibilities:
- Adhere to batch manufacturing records (BMR) and cGMP standards for granulation, blending, compression, coating, and encapsulation.
- Perform line clearance, in-process checks, and documentation.
- Supervise packing operations for primary and secondary stages, ensuring BPR and SOP compliance.
- Operate machines (RMG, FBP, Cadmech, Parle, Strip) and ensure correct material usage, labeling, and packing quality.
5. Warehouse
- Sections: RM & PM Receipt, Handling, Storage, Dispensing
- Positions: Executive, Senior Officer, Officer
- Experience: 2-8 years
- Education: B.Pharm, M.Sc., B.Sc., B.Com., D.Pharm
Responsibilities:
- Manage receipt, storage, handling, and dispensing of RM and PM per SOPs and GMP guidelines.
- Coordinate material inwarding, sampling, GRN preparation, and inventory via ERP systems.
- Follow FEFO/FIFO systems and maintain logbooks, bin cards, and stock registers.
- Ensure hygiene, pest control, and calibration of weighing balances.
Why Join Emil Pharmaceutical?
- Work in a regulated plant with a focus on quality and compliance.
- Opportunities for career growth in a dynamic pharmaceutical environment.
- Collaborate with a team committed to innovation and operational excellence.
How to Apply
Interested candidates meeting the eligibility criteria should attend the walk-in drive with their updated resume, educational certificates, experience letters, and a recent passport-sized photograph. For inquiries, contact the HR team via email at hr@emilpharma.com.
Walk-In Drive Details:
- Date: June 29, 2025
- Time: 9:00 AM to 5:30 PM
- Venue: Hotel Woodlands, 8, National Highway, Near Tejpal Motors, Pramukh Hills, Balitha, Vapi, Gujarat 396191
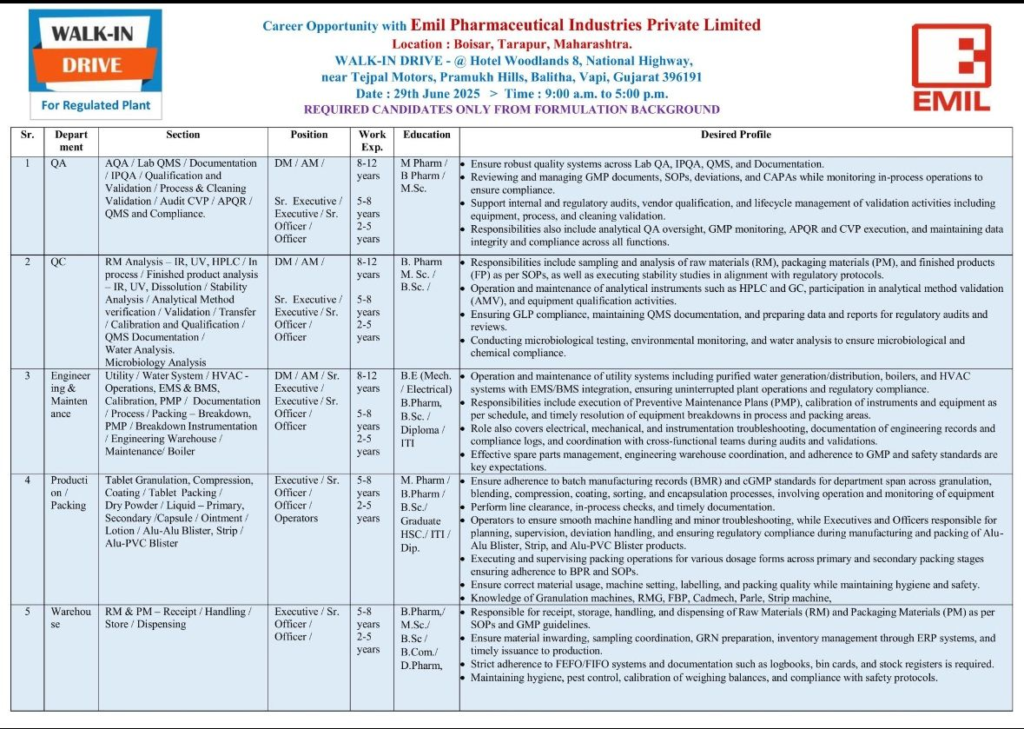
Seize this opportunity to advance your career with Emil Pharmaceutical Industries Pvt. Ltd. We look forward to meeting you!