Dishman Carbogen Amcis, a global leader in API manufacturing and contract research, is hosting a walk-in drive for its Ahmedabad facility. Join our USFDA-approved team to advance pharmaceutical innovation.
Contents
About Dishman Carbogen Amcis
Founded in 1983, Dishman Carbogen Amcis specializes in APIs, contract research, and manufacturing services. Our Ahmedabad facilities (Bavla and Naroda) are USFDA and PMDA certified. Visit Dishman Carbogen Amcis.
Current Vacancies in Ahmedabad
We are hiring for roles in Engineering, R&D, Analytical Development, Quality Control, and Quality Assurance at our Dishman Corporate House, Ahmedabad. Candidates with API experience preferred.
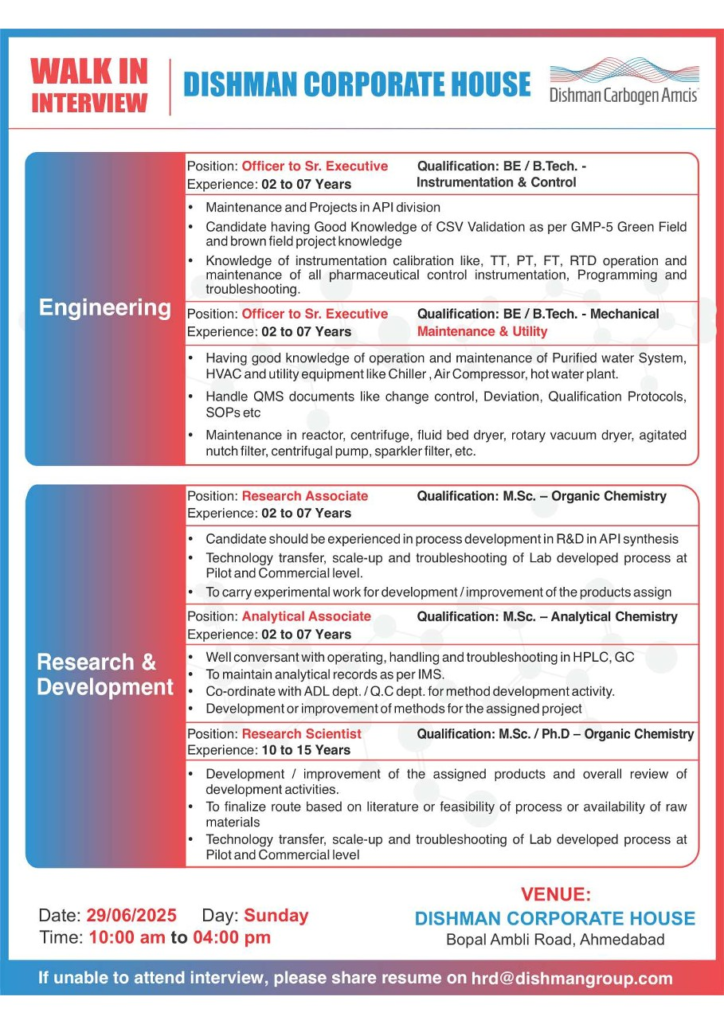
Engineering – Instrumentation & Control
Support API operations with expertise in instrumentation and CSV validation.
- Position: Officer to Sr. Executive
- Qualification: B.E./B.Tech (Instrumentation)
- Experience: 2-7 years
Skills:
- CSV validation per GAMP-5, GMP
- Green/brown field project knowledge
- Calibration and maintenance of TT, PT, FT, RTD
- PLC programming and troubleshooting
Engineering – Maintenance & Utility
Maintain critical utility systems and equipment in API manufacturing.
- Position: Officer to Sr. Executive
- Qualification: B.E./B.Tech (Mechanical)
- Experience: 2-7 years
Skills:
- Operation/maintenance of purified water systems, HVAC, chillers
- Handle reactors, centrifuges, fluid bed dryers, rotary vacuum dryers
- Manage QMS documents (change control, deviations, SOPs)
Research & Development
Drive API synthesis and process development.
Position: Research Associate
- Qualification: M.Sc. (Organic Chemistry)
- Experience: 2-7 years
Skills:
- Process development in API synthesis
- Technology transfer, scale-up, troubleshooting
Position: Research Scientist
- Qualification: M.Sc./Ph.D. (Organic Chemistry)
- Experience: 10-15 years
Skills:
- Lead product development and process improvement
- Finalize synthetic routes based on literature/feasibility
- Oversee technology transfer and scale-up
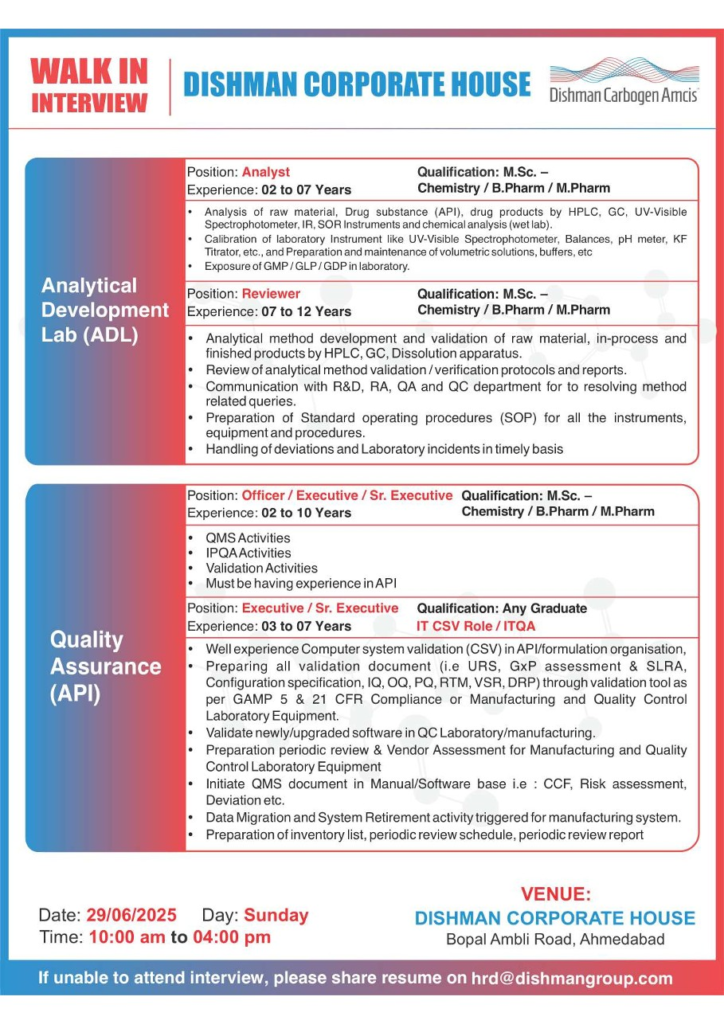
Analytical Development Lab (ADL)
Support analytical method development and quality control.
Position: Analytical Associate
- Qualification: M.Sc. (Analytical Chemistry)
- Experience: 2-7 years
Skills:
- Operate/troubleshoot HPLC, GC
- Coordinate with ADL/QC for method development
- Maintain IMS-compliant analytical records
Position: Analyst
- Qualification: M.Sc. (Chemistry), B.Pharm, M.Pharm
- Experience: 2-7 years
Skills:
- Analyze raw materials, APIs, drug products using HPLC, GC, UV-Vis, IR
- Calibrate lab instruments (UV-Vis, pH meter, KF titrator)
- Knowledge of GMP/GLP/GDP
Position: Reviewer
- Qualification: M.Sc. (Chemistry), B.Pharm, M.Pharm
- Experience: 7-12 years
Skills:
- Method development/validation using HPLC, GC, dissolution
- Review validation protocols/reports
- Handle deviations, lab incidents, SOP preparation
Quality Assurance (API)
Ensure compliance through QMS and validation activities.
Position: Officer/Executive/Sr. Executive
- Qualification: M.Sc. (Chemistry), B.Pharm, M.Pharm
- Experience: 2-10 years
Skills:
- QMS, IPQA, and validation activities
- API manufacturing experience
Position: Executive/Sr. Executive (IT CSV/ITQA)
- Qualification: Any Graduate
- Experience: 3-7 years
Skills:
- CSV validation per GAMP-5, 21 CFR
- Prepare URS, IQ, OQ, PQ, RTM, VSR
- Validate QC/manufacturing software
- Manage QMS documents (CCF, risk assessment, deviations)
Walk-In Drive Details
Join us for a walk-in interview to explore these opportunities in API manufacturing and research.
| Details | Information |
|---|---|
| Date & Time | Sunday, June 29, 2025 (10:00 AM – 4:00 PM) |
| Venue | Dishman Corporate House, Bopal Ambli Road, Ahmedabad, Gujarat |
| Documents Required | Updated Resume, Educational Certificates, Aadhar/PAN Card, Recent Increment Letter, 3 Months Payslips, Bank Statement |
| Work Location | Ahmedabad (Bavla/Naroda facilities) |
Application for Non-Attendees
If unable to attend, send your resume to hrd@dishmangroup.com.
Verified by Trusted HRs
The post is released by the Dishman Corporate House LinkedIn page. Click here to visit the post
Why Join Dishman Carbogen Amcis?
Dishman is rated 3.8/5 for work-life balance by 200+ employees in Ahmedabad. Explore pharma careers at PharmaJobs.
- Competitive roles in API manufacturing and R&D
- USFDA-approved, high-containment facilities
- Opportunities for growth in a global organization
Apply Now
Attend our walk-in drive on June 29, 2025, or email your resume to join Dishman Carbogen Amcis in Ahmedabad. Explore industry insights at BioPharmaWorld or PharmaVoice. Innovate with us!
