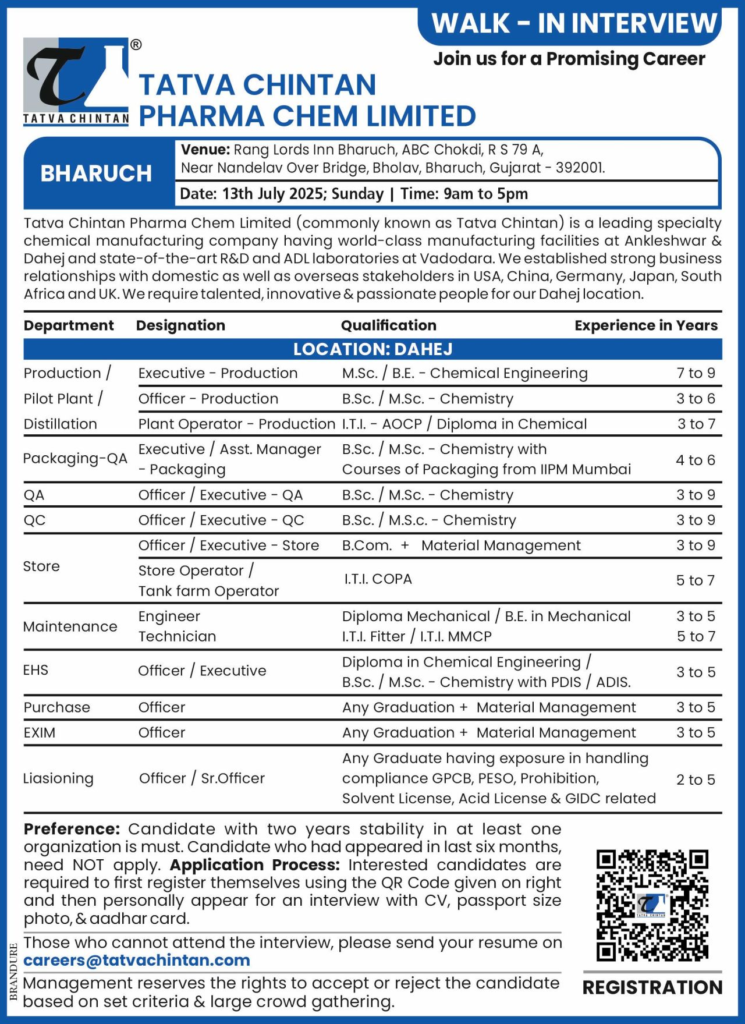
Tatva Chintan Pharma Chem Limited, a leading specialty chemical manufacturer founded in 1996, is hosting a walk-in interview at our Dahej SEZ facility in Gujarat.
With world-class manufacturing plants in Ankleshwar and Dahej, and a state-of-the-art R&D center in Vadodara, we produce over 250 products, including Phase Transfer Catalysts (PTC), Structure Directing Agents (SDA), and pharmaceutical intermediates.
Our USFDA, EDQM, and EU-GMP compliant facilities serve global clients in the USA, China, Germany, Japan, South Africa, and the UK. Join our team of 700+ employees to contribute to innovative chemical manufacturing in a dynamic, sustainable environment.
Contents
Walk-In Interview Details
- Date: Sunday, July 13, 2025
- Time: 9:00 AM to 5:00 PM
- Venue: Rang Lords Inn Bharuch, ABC Chokdi, R S 79 A, Near Nandelav Over Bridge, Bholav, Bharuch, Gujarat – 392001
- Job Location: Tatva Chintan Pharma Chem Limited, Dahej SEZ, Plot No. Z/103/F/1, Dahej SEZ, Taluka Vagra, Bharuch, Gujarat – 392130
- Registration: Candidates must pre-register using the QR code provided in the original job posting before attending.
Required Documents:
- Updated resume (2 copies)
- Passport-size photographs (2)
- Aadhar card (original and photocopy)
- Educational certificates (original and photocopies)
- Last 3 months’ payslips and latest increment letter
Note:
- Candidates must have at least two years of stability in one organization.
- Candidates interviewed at Tatva Chintan in the last 6 months are ineligible.
- Preference for candidates with experience in distillation plants or regulated environments (USFDA, EDQM).
- Management reserves the right to accept or reject candidates based on set criteria.
Open Positions
Production / Pilot Plant / Distillation
Executive Production
- Qualification: M.Sc / B.E Chemical Engineering
- Experience: 7–9 years
- Responsibilities: Supervise production processes (reactors, distillation columns, filters), ensure cGMP compliance, manage DCS operations, and support audits.
- Key Skills: Expertise in distillation plants, DCS operations, and regulatory compliance.
Officer Production
- Qualification: B.Sc / M.Sc Chemistry
- Experience: 3–6 years
- Responsibilities: Monitor production processes, maintain Batch Manufacturing Records (BMR), and ensure safety and cGMP standards.
- Key Skills: Knowledge of chemical processes and regulatory documentation.
Plant Operator Production
- Qualification: ITI AOCP / Diploma in Chemical Engineering
- Experience: 3–7 years
- Responsibilities: Operate equipment (reactors, centrifuges, dryers), perform routine checks, and adhere to SOPs.
- Key Skills: Hands-on experience in chemical plant operations.
Packaging – QA
Executive / Assistant Manager Packaging
- Qualification: B.Sc / M.Sc Chemistry with Packaging Course from IIPM Mumbai
- Experience: 4–6 years
- Responsibilities: Oversee packaging quality, validate packaging processes, and ensure compliance with regulatory standards.
- Key Skills: Knowledge of packaging standards and cGMP.
Quality Assurance (QA)
Officer / Executive QA
- Qualification: B.Sc / M.Sc Chemistry
- Experience: 3–9 years
- Responsibilities: Conduct in-process quality checks, review BMR, manage deviations, and support audits (USFDA, EDQM).
- Key Skills: Strong understanding of cGMP, GDP, and regulatory compliance.
Quality Control (QC)
Officer / Executive QC
- Qualification: B.Sc / M.Sc Chemistry
- Experience: 3–9 years
- Responsibilities: Perform analytical testing (HPLC, GC, KF Titrator), conduct method validation, and maintain GLP compliance.
- Key Skills: Proficiency in QC instruments and regulatory documentation.
Store
Officer / Executive Store
- Qualification: B.Com + Material Management
- Experience: 3–9 years
- Responsibilities: Manage raw material and finished goods inventory, handle SAP operations, and ensure cGMP-compliant documentation.
- Key Skills: Experience in store management and SAP.
Store Operator / Tank Farm Operator
- Qualification: ITI COPA
- Experience: 5–7 years
- Responsibilities: Handle material storage, tank farm operations, and inventory tracking per SOPs.
- Key Skills: Knowledge of tank farm operations and safety protocols.
Maintenance
Engineer Technician
- Qualification: Diploma Mechanical / B.E Mechanical / ITI Fitter / ITI MMCP
- Experience: 3–5 years
- Responsibilities: Perform preventive and breakdown maintenance on reactors, pumps, and utilities, ensuring cGMP compliance.
- Key Skills: Expertise in mechanical maintenance and regulatory standards.
Environment, Health, and Safety (EHS)
Officer / Executive EHS
- Qualification: Diploma in Chemical Engineering / B.Sc / M.Sc Chemistry with PDIS / ADIS
- Experience: 3–5 years
- Responsibilities: Implement EHS policies, conduct risk assessments, manage ETP/MEE operations, and ensure regulatory compliance.
- Key Skills: Knowledge of EHS regulations, HAZOP, and ETP operations.
Purchase
Officer Purchase
- Qualification: Any Graduation + Material Management
- Experience: 3–5 years
- Responsibilities: Manage procurement of raw materials, negotiate with vendors, and maintain purchase records per cGMP standards.
- Key Skills: Procurement expertise and vendor management.
EXIM (Export-Import)
Officer EXIM
- Qualification: Any Graduation + Material Management
- Experience: 3–5 years
- Responsibilities: Handle export-import documentation, coordinate with customs, and ensure compliance with international trade regulations.
- Key Skills: Knowledge of EXIM procedures and regulatory compliance.
Liaisoning
Officer / Senior Officer Liaisoning
- Qualification: Any Graduate with exposure to GPCB, PESO, Prohibition, Solvent License, and GIDC compliance
- Experience: 2–5 years
- Responsibilities: Manage regulatory compliances (GPCB, PESO, GIDC), coordinate with government agencies, and maintain licenses.
- Key Skills: Strong liaisoning skills and knowledge of regulatory frameworks.
Why Join Tatva Chintan?
- Global Leader: Work with a leading producer of PTC, the world’s second-largest manufacturer of SDAs, and India’s largest producer of electrolyte salts for supercapacitor batteries, serving clients like Merck, Bayer, and SRF.
- Innovative Environment: Contribute to cutting-edge chemical manufacturing at a facility with a 48,000 TPA capacity, supported by a 36,000 sq. ft. DSIR-recognized R&D center in Vadodara.
- Regulatory Excellence: Gain experience in USFDA, EDQM, and EU-GMP compliant operations, ensuring high-quality standards.
- Work-Life Balance: Rated 3.5/5 on AmbitionBox for work-life balance, with 71% of employees giving a 4+ rating, though career growth is moderate at 3.2/5.
How to Apply
- Walk-In: Attend the interview on July 13, 2025, at Rang Lords Inn Bharuch with all required documents after pre-registering via the QR code.
- For Those Unable to Attend: Email your updated CV to careers@tatvachintan.com, mentioning the specific role and department (e.g., “Executive Production”) in the subject line. Include total experience, current CTC, expected CTC, and notice period.
- Contact: For inquiries, email careers@tatvachintan.com or call +91 75730 46951.
Verified by Trusted HRs
The post is released by the Tatva Chintan Pharma Chem Limited LinkedIn page. Click here to visit the post
Note: Candidates with distillation plant experience and regulatory exposure are preferred.
About Tatva Chintan Pharma Chem
Founded in 1996 by Chintan Shah, Ajay Patel, and Shekhar Somani, Tatva Chintan Pharma Chem Limited is headquartered in Vadodara, Gujarat, with manufacturing facilities in Ankleshwar and Dahej SEZ.
Specializing in specialty chemicals like PTC, SDAs, and pharmaceutical intermediates, we export to 25+ countries, with 76% of revenue from exports in FY 2020. Our Dahej facility features advanced equipment and a pilot plant, while our Vadodara R&D center supports innovation with continuous flow reactors and high-pressure autoclaves. Learn more at www.tatvachintan.com.
Important Disclaimer
Tatva Chintan Pharma Chem Limited does not charge any fees for recruitment or authorize agencies to collect payments. Report suspicious job offers to cs@tatvachintan.com.
Join Tatva Chintan and contribute to innovative, sustainable specialty chemical manufacturing in a globally recognized organization!


