Piramal Pharma Limited, a globally recognized Contract Development and Manufacturing Organization (CDMO), invites candidates for a walk-in interview at our Pithampur, Indore plant. Join our dynamic team to contribute to pharmaceutical excellence in a USFDA and MHRA-compliant facility.
Contents
Why Join Piramal Pharma?
Piramal Pharma Solutions, a division of Piramal Enterprises Limited, operates across North America, Europe, and Asia, offering end-to-end services in drug development and manufacturing.
Our Pithampur facility, with a 4.5 billion unit/year OSD capacity, is accredited by USFDA, EU GMP, and other global regulators, providing a robust platform for career growth.
Job Opportunities at Pithampur Plant
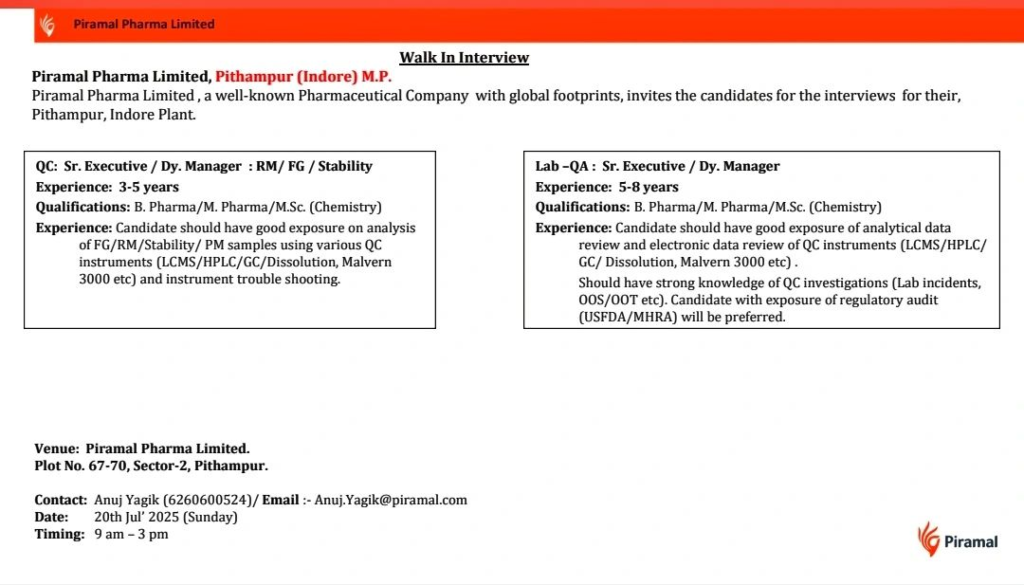
We are hiring for Quality Control (QC) and Lab Quality Assurance (Lab-QA) roles at our Pithampur facility, focusing on oral solid dosage (OSD) and analytical excellence.
Open Positions
QC: Sr. Executive / Deputy Manager (RM/FG/Stability)
- Experience: 3-5 years
- Qualification: B.Pharm, M.Pharm, M.Sc. (Chemistry)
- Responsibilities: Perform accurate analysis, ensure data integrity, and support stability studies per ICH guidelines.
Skills Required:
- Expertise in analysis of Raw Material (RM), Finished Goods (FG), Stability, and Packaging Material (PM) samples using QC instruments (LCMS, HPLC, GC, Dissolution, Malvern 3000, etc.).
- Proficiency in instrument troubleshooting.
Lab-QA: Sr. Executive / Deputy Manager
- Experience: 5-8 years
- Qualification: B.Pharm, M.Pharm, M.Sc. (Chemistry)
Skills Required:
- Strong experience in analytical data review and electronic data review of QC instruments (LCMS, HPLC, GC, Dissolution, Malvern 3000, etc.).
- In-depth knowledge of QC investigations (Lab incidents, OOS/OOT, etc.).
- Exposure to regulatory audits (USFDA, MHRA) preferred.
- Responsibilities: Oversee analytical data integrity, manage QC investigations, and ensure compliance with global regulatory standards.
Walk-In Interview Details
- Date: 20th July 2025 (Sunday)
- Time: 9:00 AM to 3:00 PM
- Venue: Piramal Pharma Limited, Plot No. 67-70, Sector-2, Pithampur, Indore, Madhya Pradesh – 454775
- Contact: Anuj Yagik (+91 6260600524) | Email: Anuj.Yagik@piramal.com
Requirements:
- Updated resume
- Educational certificates
- Government ID proof (Aadhar/PAN card)
- Passport-size photographs (3)
- Latest salary proof (payslips, appointment letters)
Unable to Attend?
Email your resume to Anuj.Yagik@piramal.com. Explore more opportunities at Piramal Pharma Careers.
Why Work at Piramal’s Pithampur Facility?
Our Pithampur plant is a state-of-the-art facility specializing in OSD manufacturing and packaging, with advanced analytical labs conducting 99.5% of testing in-house.
Employees benefit from a 3.6/5 rated work culture, exposure to global regulatory standards, and access to Piramal Learning University for skill.
How to Prepare for the Interview
- Update your resume with relevant QC or QA experience, emphasizing instrument expertise (LCMS, HPLC, etc.) and regulatory knowledge.
- Bring all required documents, including ID and salary proofs.
- Be prepared to discuss your experience with analytical data review, QC investigations, or regulatory audits.
About Piramal Pharma Limited
Piramal Pharma Limited, part of the Piramal Group, is a leading CDMO with operations in 30 countries and a turnover of ~$1.9 billion in FY 2022. Our Pithampur facility supports clinical, pilot, and commercial-scale OSD manufacturing, with accreditations from USFDA, EU GMP, and more.
Career Growth Opportunities
With a 3.8/5 employee rating, Piramal Pharma offers a supportive environment and opportunities for internal mobility, such as transitions to regulatory affairs or validation roles. Our focus on learning ensures continuous professional development.
Contact Us
- Email: Anuj.Yagik@piramal.com
- Phone: +91 6260600524
- Website: www.piramalpharmasolutions.com
Apply Today!
Join Piramal Pharma Limited and contribute to global healthcare excellence. Attend the walk-in interview on 20th July 2025 in Pithampur, Indore, or apply online. Take your career to the next level with a trusted pharmaceutical leader! #SeekToTransform


