Ciron Drugs & Pharmaceuticals Pvt. Ltd., a leader in tablet and capsule formulation, is hosting a walk-in drive at our Palghar plant. Join our team to contribute to high-quality pharmaceutical production in a dynamic environment.
Contents
Why Ciron Drugs & Pharmaceuticals?
Our Palghar facility is equipped for advanced formulation manufacturing, offering competitive salaries and career growth. We value expertise in quality assurance and control, fostering innovation in a cGMP-compliant setting for professionals passionate about healthcare.
Walk-In Drive Details
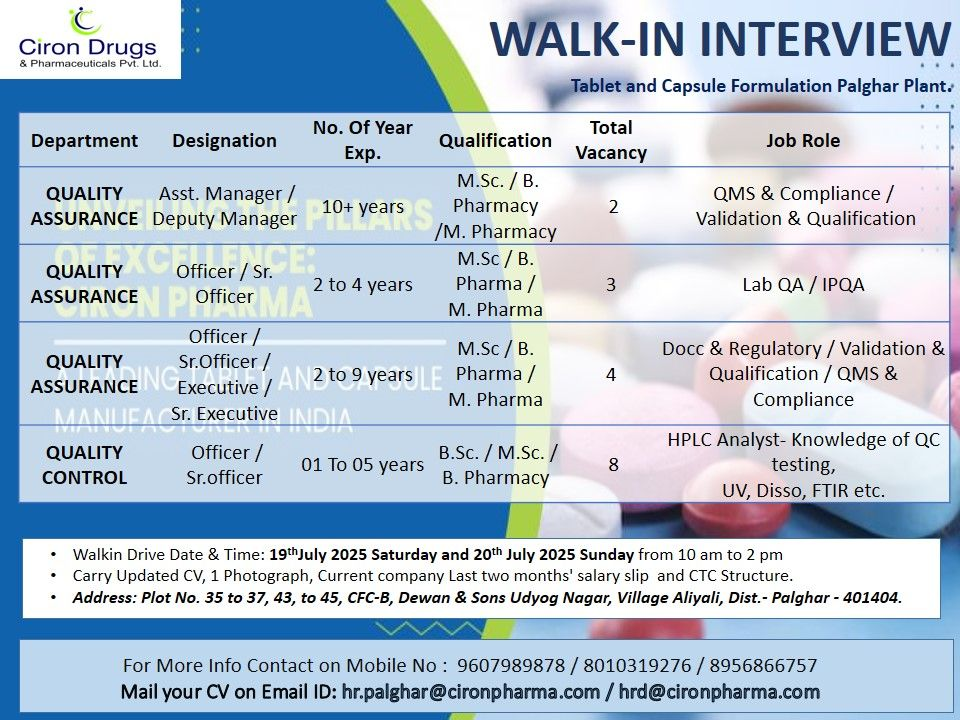
- Date: July 19-20, 2025 (Saturday-Sunday)
- Time: 10:00 AM to 2:00 PM
- Venue: Plot No. 35-37, 43-45, CFC-B, Dewan & Sons Udyog Nagar, Village Aliyali, Palghar – 401404
- Note: Immediate joiners preferred. Only candidates with relevant experience should apply.
Documents Required
- Updated CV
- One photograph
- Last two months’ salary slips
- Current company CTC structure
Email resumes to hr.palghar@cironpharma.com or hrd@cironpharma.com if unable to attend. Contact: 9607989878, 8010319276, 8956866757.
Open Positions
We are hiring for Quality Assurance and Quality Control roles at our Palghar plant. Candidates must have hands-on experience in pharmaceutical formulation and be familiar with cGMP standards.
Quality Assurance
Asst. Manager/Deputy Manager
- Experience: 10+ years
- Qualification: M.Sc., B.Pharm, M.Pharm
- Vacancies: 2
- Job Role: Manage QMS, compliance, validation, and qualification processes; ensure regulatory adherence.
Officer/Sr. Officer (Lab QA/IPQA)
- Experience: 2-4 years
- Qualification: M.Sc., B.Pharm, M.Pharm
- Vacancies: 3
- Job Role: Conduct IPQA checks, manage lab QA, ensure process compliance.
Officer/Sr. Officer/Executive/Sr. Executive
- Experience: 2-9 years
- Qualification: M.Sc., B.Pharm, M.Pharm
- Vacancies: 4
- Job Role: Handle documentation, regulatory tasks, validation, qualification, and QMS compliance.
Quality Control
Officer/Sr. Officer
- Experience: 1-5 years
- Qualification: B.Sc., M.Sc., B.Pharm
- Vacancies: 8
- Job Role: Perform HPLC analysis, UV, dissolution, FTIR testing; ensure QC compliance.
How to Prepare
Highlight your experience in tablet/capsule formulation and cGMP compliance on your CV. Bring all required documents in hard copy. Familiarity with HPLC, UV, and QMS processes will strengthen your application.
Why Palghar?
Located in a growing industrial hub near Mumbai, our Palghar plant offers excellent connectivity and career opportunities in pharmaceutical manufacturing, making it ideal for professionals seeking growth.
Join Our Mission
Ciron Drugs & Pharmaceuticals is dedicated to delivering high-quality formulations globally. Join us to drive innovation in healthcare while advancing your career. Visit www.cironpharma.com for more details.
Apply now to advance your career with Ciron Drugs & Pharmaceuticals!


