Damaira Pharmaceuticals Pvt. Ltd., an emerging leader in EU and USFDA-compliant carbapenem dry powder injectable manufacturing, invites talented professionals to our walk-in interview in Panchkula, Haryana. Join our innovative team dedicated to revolutionizing the pharmaceutical industry with high-quality antibiotics.
Contents
Why Damaira Pharmaceuticals?
Established in 2020, Damaira operates a state-of-the-art facility in Panchkula, specializing in carbapenem injections like Meropenem and Imipenem-Cilastatin. Equipped with advanced SCADA 21 CFR systems, we ensure precision and compliance with global standards, offering a dynamic environment for career growth.
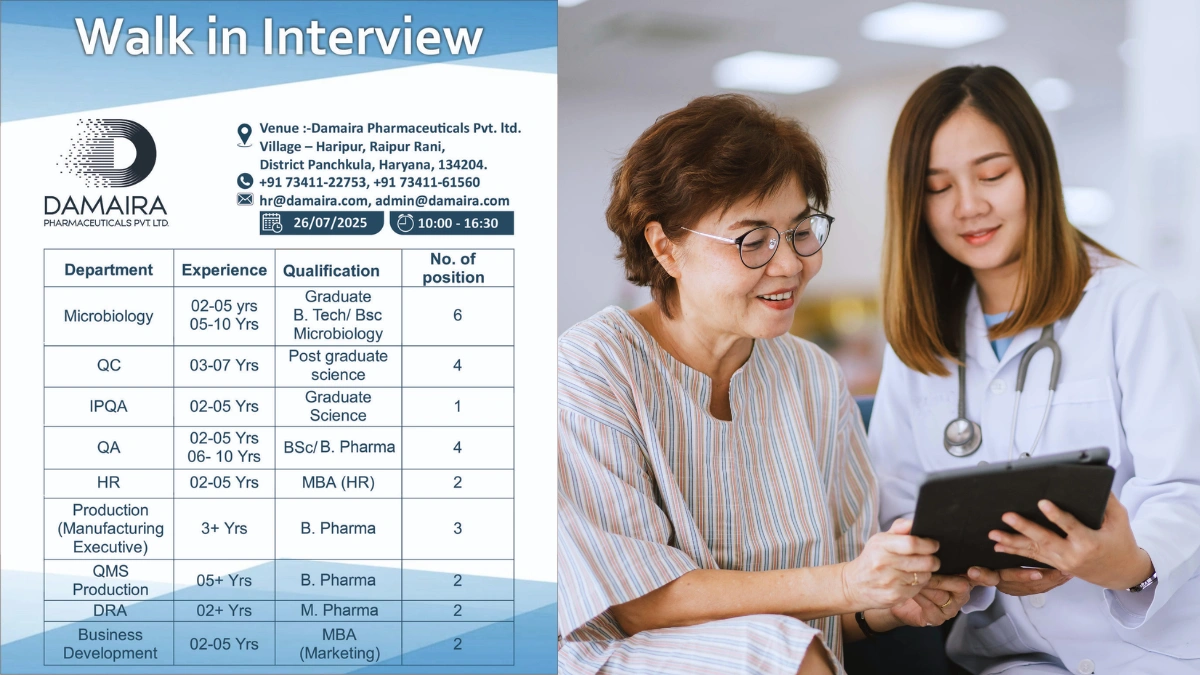
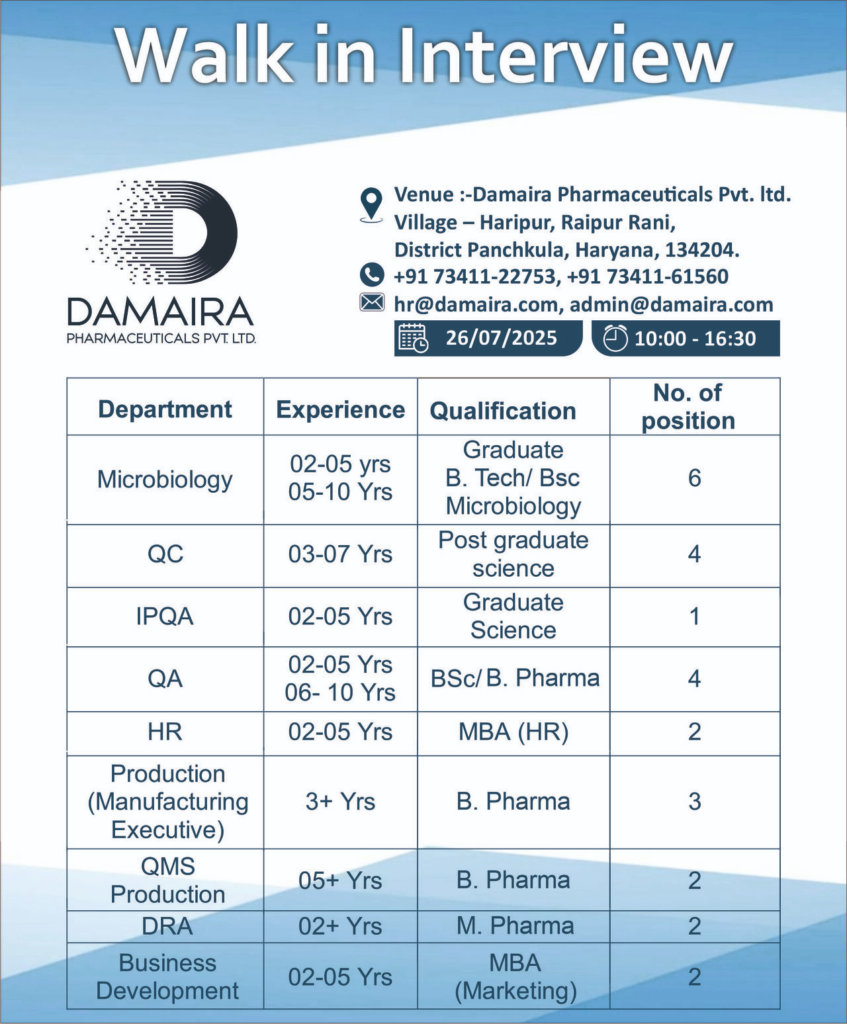
Walk-In Interview Details
Date: 26th July 2025 (Saturday)
Time: 10:00 AM to 4:30 PM
Venue: Damaira Pharmaceuticals Pvt. Ltd., Village Haripur, Raipur Rani, District Panchkula, Haryana – 134204
Contact: +91 73411-22753, +91 73411-61560, or email hr@damaira.com, admin@damaira.com
Requirements: Bring updated CV, educational certificates, experience letters, and Aadhar card.
Note: Candidates must have experience in pharmaceutical manufacturing, preferably in a regulatory-approved environment (USFDA/EU).
Available Positions – Panchkula (Carbapenem Dry Powder Injectable Facility)
Microbiology
- Experience: 2-5 years (6 positions), 5-10 years (2 positions)
- Qualification: B.Tech / B.Sc (Microbiology)
- No. of Positions: 6
- Skills: Microbiological testing, environmental monitoring, sterility, and compliance with cGMP standards.
Quality Control (QC)
- Experience: 3-7 years
- Qualification: Postgraduate (Science)
- No. of Positions: 4
- Skills: Testing raw materials, intermediates, and finished products; expertise in HPLC, GC, or LCMS preferred.
In-Process Quality Assurance (IPQA)
- Experience: 2-5 years
- Qualification: Graduate (Science)
- No. of Positions: 1
- Skills: In-process checks, documentation, and adherence to cGMP in sterile manufacturing.
Quality Assurance (QA)
- Experience: 2-5 years (2 positions), 6-10 years (2 positions)
- Qualification: B.Sc / B.Pharm
- No. of Positions: 4
- Skills: QMS, equipment validation, cleaning validation, and regulatory compliance.
Human Resources (HR)
- Experience: 2-5 years
- Qualification: MBA (HR)
- No. of Positions: 2
- Skills: Recruitment, employee engagement, and compliance with labor regulations in a pharma setting.
Production (Manufacturing Executive)
- Experience: 3+ years
- Qualification: B.Pharm
- No. of Positions: 3
- Skills: Carbapenem production, handling reactors, and compliance with SOPs in a sterile environment.
QMS Production
- Experience: 5+ years
- Qualification: B.Pharm
- No. of Positions: 2
- Skills: Managing QMS for production, process validation, and ensuring regulatory compliance.
Drug Regulatory Affairs (DRA)
- Experience: 2+ years
- Qualification: M.Pharm
- No. of Positions: 2
- Skills: Dossier preparation (CTD/eCTD), regulatory submissions, and compliance with USFDA/EU norms.
Business Development
- Experience: 2-5 years
- Qualification: MBA (Marketing)
- No. of Positions: 2
- Skills: Market analysis, client relationship management, and promoting carbapenem products globally.
Why These Roles Matter
These positions are critical for maintaining Damaira’s high standards in carbapenem manufacturing. From microbiology to regulatory affairs, your expertise will ensure quality and compliance, supporting our mission to combat severe bacterial infections. Learn more about carbapenem antibiotics.
How to Prepare for the Interview
- Bring updated CV, certificates, and experience proof.
- Highlight relevant experience in sterile manufacturing or regulatory environments.
- Be prepared to discuss expertise in cGMP, QMS, or regulatory submissions.
- Email CV to hr@damaira.com if unable to attend.
Why Panchkula?
Panchkula is a growing pharmaceutical hub in Haryana, offering excellent career prospects. Damaira’s EU/USFDA-compliant facility in Haripur provides a cutting-edge platform for professionals. Explore Panchkula’s industrial landscape.
Join Our Team
Damaira Pharmaceuticals is poised to revolutionize the pharma industry with its focus on carbapenem injectables. This walk-in interview is your chance to join a forward-thinking company. Visit Damaira Pharmaceuticals for more details. Don’t miss this opportunity to shape your career!