Join Endo India Par Formulations, a leading pharmaceutical company dedicated to developing innovative, safe, and cost-effective pharmaceuticals. We are hosting a walk-in interview in Hyderabad for talented professionals in microbiology and laboratory operations. Discover rewarding career paths with long-term growth potential at Endo!
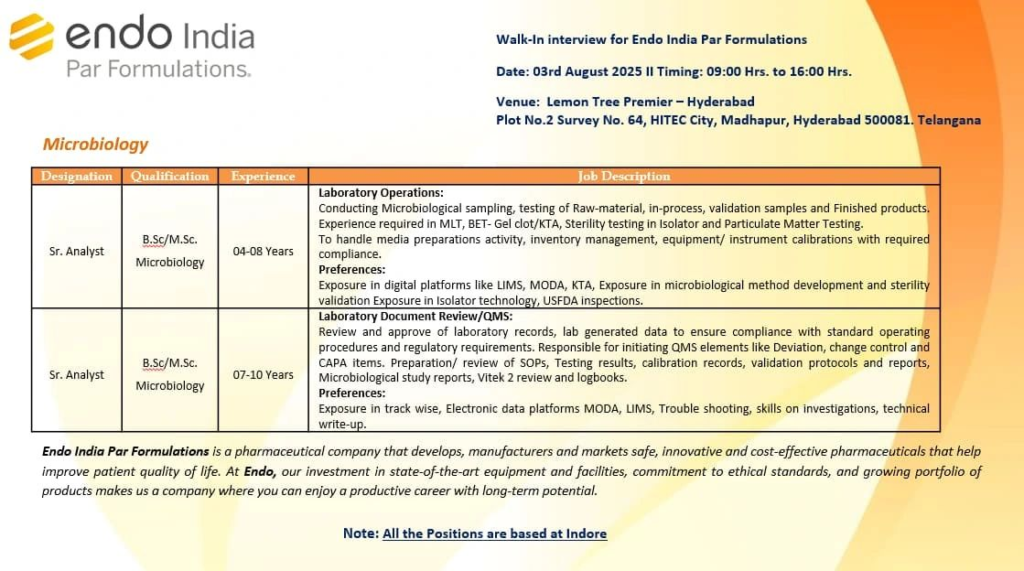
Walk-In Interview Details
- Date: August 3, 2025
- Time: 9:00 AM to 4:00 PM
- Venue: Lemon Tree Premier, Hyderabad
Plot No. 2, Survey No. 64, HITEC City, Madhapur, Hyderabad 500081, Telangana - Note: All positions are based in Indore.
Why Join Endo India Par Formulations?
Endo India Par Formulations is committed to improving patient quality of life through cutting-edge pharmaceutical solutions. Our state-of-the-art facilities, ethical standards, and innovative product portfolio create an environment where professionals thrive. Explore a fulfilling career in a company that values excellence and growth.
About Endo India
Endo India is a trusted name in the pharmaceutical industry, specializing in high-quality drug development and manufacturing. With a focus on innovation and compliance, we offer a dynamic workplace for professionals passionate about making a difference in healthcare.
Job Openings in Microbiology
We are seeking skilled individuals for various roles in microbiology and laboratory operations. Below are the details of the open positions:
| Designation | Qualification | Experience | Location |
|---|---|---|---|
| Sr. Analyst | B.Sc./M.Sc. Microbiology | 4–8 Years | Indore |
| Sr. Analyst | B.Sc./M.Sc. Microbiology | 7–10 Years | Indore |
| Analyst | B.Sc./M.Sc. Microbiology | 2–6 Years | Indore |
1. Senior Analyst – Laboratory Operations
Job Responsibilities
- Conduct microbiological sampling and testing of raw materials, in-process samples, validation samples, and finished products.
- Perform MLT, BET (Gel Clot/KTA), sterility testing in isolators, and particulate matter testing.
- Manage media preparation, inventory, and equipment/instrument calibration with compliance to regulatory standards.
- Ensure adherence to Good Manufacturing Practices (GMP) and USFDA regulations.
Preferred Skills
- Experience with digital platforms like LIMS, MODA, and KTA.
- Expertise in microbiological method development and sterility validation.
- Familiarity with isolator technology and USFDA inspections.
2. Senior Analyst – Laboratory Document Review/QMS
Job Responsibilities
- Review and approve laboratory records and data for compliance with SOPs and regulatory standards.
- Initiate and manage QMS elements such as deviations, change controls, and CAPA items.
- Prepare and review SOPs, testing results, calibration records, validation protocols, and microbiological study reports.
- Maintain Vitek 2 reviews and logbooks.
Preferred Skills
- Proficiency in TrackWise and electronic data platforms like MODA and LIMS.
- Strong troubleshooting and investigation skills.
- Expertise in technical writing and regulatory compliance.
3. Analyst – Environmental Monitoring
Job Responsibilities
- Execute microbial environmental monitoring in manufacturing environments using passive/active air sampling and surface monitoring.
- Evaluate cleanliness levels and ensure compliance with aseptic standards.
- Support aseptic process simulations and maintain knowledge of aseptic behavior.
Preferred Skills
- Exposure to digital platforms like MODA.
- Experience with isolator technology and aseptic process simulations.
- Knowledge of USFDA inspection standards.
Why Work with Endo India?
- Innovative Environment: Access state-of-the-art facilities and cutting-edge technology.
- Career Growth: Opportunities for professional development and long-term career advancement.
- Ethical Standards: Join a company committed to integrity and quality in pharmaceuticals.
- Impactful Work: Contribute to improving patient lives through safe and effective products.
How to Prepare for the Walk-In Interview
- Bring your updated resume and relevant certifications.
- Be prepared to discuss your experience in microbiology, laboratory operations, or QMS.
- Familiarize yourself with regulatory standards like USFDA and GMP.
- Highlight your expertise in digital platforms like LIMS, MODA, or TrackWise.

About the Venue
The walk-in interview will be held at Lemon Tree Premier, Hyderabad, a premier venue in the heart of HITEC City. Easily accessible, it offers a professional setting for candidates to showcase their skills.
Join Our Mission
At Endo India Par Formulations, we believe in creating a healthier future through innovation and excellence. If you’re passionate about microbiology, laboratory operations, or quality management, this is your chance to shine. Attend our walk-in interview on August 3, 2025, and take the first step toward a rewarding career!
For more information about Endo India, visit Endo’s official website. To learn about career opportunities in pharmaceuticals, check pharmaceutical job trends.

