Discover pharma jobs at V-Ensure Pharma Technologies! Walk-in interview for quality assurance and production roles in Raigad, Maharashtra, on 27th September 2025. Apply now!
Contents
About V-Ensure Pharma Technologies
V-Ensure Pharma Technologies Pvt. Ltd., founded in 2010, is a leading Contract Development and Manufacturing Organization (CDMO) specializing in pharmaceutical formulations for regulated markets.
With USFDA and EU-GMP approved facilities, the company excels in developing complex generics, oral solid dosage forms, and innovative drug products. Headquartered in Navi Mumbai, Maharashtra, V-Ensure emphasizes scientific innovation, regulatory compliance, and employee growth, making it a top choice for pharmaceutical careers in India.
Job Details
- Company Name: V-Ensure Pharma Technologies Pvt. Ltd.
- Experience: 2–6 years
- Qualification: B.Pharm/M.Pharm, M.Sc., Any Graduate/Under Graduate/Diploma, D.Pharm/ITI/B.Sc.
- Location: N-32, Additional Patalganga MIDC, Tal. Panvel, Dist. Raigad, Maharashtra
- Work Type: Full-time (Male candidates only for QA QMS role)
Job Description
V-Ensure Pharma Technologies is hiring for key positions in Quality Assurance and Production departments at its OSD manufacturing facility. These roles suit growth-oriented professionals with 2–6 years of experience in regulated markets, focusing on compliance and efficient operations.
Quality Assurance – QMS Section
- Department: Quality Assurance
- Role: Officer
- Experience: 2–5 years (Male candidates only)
- Education/Qualification: B.Pharm/M.Pharm
Quality Assurance – ALQA/Finished/Stability/Method Validation/Lab QA/GLP Document Control
- Department: Quality Assurance
- Role: Officer/Sr. Officer/Executive
- Experience: 2–6 years
- Education/Qualification: M.Sc./B.Pharm/M.Pharm
Production – BQS Operator
- Department: Production
- Role: BQS Operator
- Experience: 2–4 years
- Education/Qualification: Any Graduate/Under Graduate/Diploma
Production – Granulation
- Department: Production
- Role: Operator/Sr. Operator
- Experience: 3–4 years
- Education/Qualification: D.Pharm/ITI/B.Sc.
Skills/Qualifications
- B.Pharm/M.Pharm or M.Sc. for QA roles with knowledge of cGMP, GDP, and data integrity
- Experience in QMS coordination, including change control, deviation, and CAPA
- Proficiency in method validation, stability protocols, and document review
- Hands-on skills in equipment handling like Blister Packing Machine (Antras, Pam pack)
- Familiarity with granulation, compression, coating, and cleaning validation for production roles
- Diploma/ITI/B.Sc. for operator positions with focus on equipment calibration and documentation
Key Responsibilities
- Coordinate QMS activities like change control, deviations, CAPA, and training management
- Review SOPs, protocols for method validation, stability, calibration, and qualification
- Analyze raw material specifications, stability data, COA, and electronic audit trails
- Perform daily manufacturing and packing area activities, including primary/secondary packing
- Handle equipment like HSMG-250, FBP, Wurster coater, blender, multimill, and comill
- Execute cleaning activities, weighing balance calibration, and maintain production log books
Benefits/Perks
- Opportunities for career growth in a USFDA/EU-GMP approved facility
- Exposure to regulated markets and innovative OSD manufacturing
- Supportive environment fostering scientific innovation and compliance
- Competitive salary and professional development programs
- Contribution to global healthcare through complex generics development
How to Apply
Walk-in with required documents for immediate interview. For more pharma job opportunities, visit Pharma Recruiter.
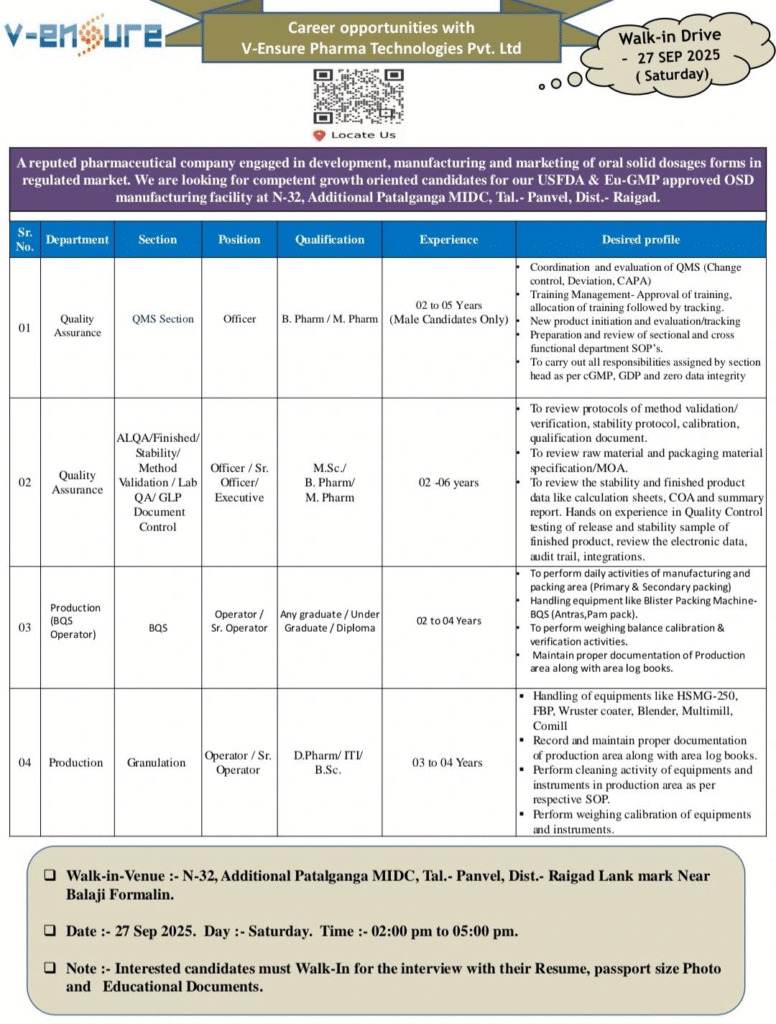
Walk-in Interview Details
- Date: 27th September 2025 (Saturday)
- Time: 02:00 PM to 05:00 PM
- Venue: N-32, Additional Patalganga MIDC, Tal. Panvel, Dist. Raigad (Landmark: Near Balaji Formalin)
Documents to Carry:
- Updated Resume
- Passport size Photo
- Educational Documents
Why You Should Join V-Ensure Pharma Technologies
V-Ensure Pharma Technologies provides a dynamic platform for professionals in pharmaceutical manufacturing and quality assurance. With a focus on innovation, regulatory excellence, and employee aspirations, it offers long-term stability and growth in pharmaceutical careers in India.
Join a team driving complex generics for global markets and thrive in a collaborative culture.
FAQs
Q1: Who can apply for these pharma jobs at V-Ensure?
A: Candidates with 2–6 years of experience and qualifications like B.Pharm/M.Pharm, M.Sc., or Diploma/ITI/B.Sc. in QA and production roles.
Q2: What documents are required for the walk-in interview?
A: Bring an updated resume, passport-size photo, and educational documents.
Q3: Are there any specific eligibility criteria?
A: Male candidates only for the QA QMS Officer role; all roles require experience in regulated markets.
Q4: What is the focus of V-Ensure’s manufacturing facility?
A: Oral solid dosage forms (OSD) for USFDA and EU-GMP approved regulated markets.
