Exciting pharma jobs in Ahmedabad! Walk-in interview on October 4, 2025, for OSD facility roles in production, QC, QA, ADL, engineering, and IT. Ideal for experienced professionals in USFDA-approved environments.
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Production/Packing – Compression/Granulation Area
- 3.2 Production/Packing – Operator (Compression/Blister Packing)
- 3.3 Production/Packing – Lead GLP Section/Lead RM/PM Section
- 3.4 QC
- 3.5 QA
- 3.6 QA – FTE (Vendor Qualification, Audit & Compliance)
- 3.7 QA – Manager/Asst. Manager (Reviewer and Team Handling)
- 3.8 ADL (AMV/AMD/Routine Analysis)
- 3.9 ADL (Documentation on ADL)
- 3.10 Engineering (Boiler Operator)
- 3.11 IT
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
STALLion Laboratories Pvt. Ltd., founded in 1988, is a leading pharmaceutical formulation manufacturer specializing in niche products. With WHO GMP-approved facilities and GLP-qualified quality control, the company excels in oral solid dosage (OSD) forms.
Unit-II, a USFDA and UK MHRA accredited OSD facility in Ahmedabad, emphasizes creativity, quality, and expansion into regulated markets like the EU.
Job Details
- Company Name: STALLion Laboratories Pvt. Ltd.
- Experience: 0-14 years (role-specific; USFDA OSD facility experience required)
- Qualification: B.Pharm, M.Pharm, M.Sc, ITI/Diploma, MCA/M.Sc-IT
- Location: Gallops Industrial Park-2, Sarkhej-Bavla Road, Dist-Ahmedabad 382220, Gujarat
- Work Type: Full-time
Job Description
STALLion Laboratories is conducting a walk-in interview for key positions at its USFDA-approved OSD Unit-II facility. These pharma jobs focus on production, quality, and support functions, requiring hands-on experience in regulated OSD environments. Preferred for local candidates passionate about pharmaceutical careers in Gujarat.
Production/Packing – Compression/Granulation Area
- Department: Production/Packing
- Role: Handle compression and granulation processes in OSD manufacturing.
- Experience: 6-10 years in USFDA OSD facility.
- Education/Qualification: B.Pharm/M.Pharm/M.Sc.
Production/Packing – Operator (Compression/Blister Packing)
- Department: Production/Packing
- Role: Operate compression and blister packing equipment.
- Experience: 2-5 years in USFDA OSD facility.
- Education/Qualification: ITI/Diploma.
Production/Packing – Lead GLP Section/Lead RM/PM Section
- Department: Production/Packing
- Role: Lead GLP, raw material, and primary material sections.
- Experience: 10-14 years in USFDA OSD facility.
- Education/Qualification: Not specified.
QC
- Department: QC
- Role: Perform HPLC analysis and quality testing.
- Experience: 2-5 years in USFDA OSD facility.
- Education/Qualification: M.Sc/M.Pharm/B.Pharm.
QA
- Department: QA
- Role: Testing, GC, GLP, computer operations, documentation, washing.
- Experience: 2-5 years in USFDA OSD facility.
- Education/Qualification: Not specified.
QA – FTE (Vendor Qualification, Audit & Compliance)
- Department: QA
- Role: Handle vendor qualification, audits, compliance, QMS, training, documentation.
- Experience: 2-5 years in USFDA OSD facility.
- Education/Qualification: M.Pharm/B.Pharm.
QA – Manager/Asst. Manager (Reviewer and Team Handling)
- Department: QA
- Role: Review documentation and manage teams.
- Experience: 8-12 years in USFDA OSD facility.
- Education/Qualification: Not specified.
ADL (AMV/AMD/Routine Analysis)
- Department: ADL
- Role: Conduct analytical method validation and routine analysis.
- Experience: 1-3 years in USFDA OSD facility.
- Education/Qualification: M.Sc/M.Pharm/B.Pharm.
ADL (Documentation on ADL)
- Department: ADL
- Role: Manage documentation for analytical development lab.
- Experience: 5-8 years in USFDA OSD facility.
- Education/Qualification: M.Sc/M.Pharm/B.Pharm.
Engineering (Boiler Operator)
- Department: Engineering
- Role: Operate and maintain boilers.
- Experience: 2-5 years in USFDA OSD facility.
- Education/Qualification: ITI/Diploma.
IT
- Department: IT
- Role: Support digitalization, implement PharmaCloud PC 360 and other software.
- Experience: 0-1 years.
- Education/Qualification: MCA/M.Sc-IT.
Skills/Qualifications
- Expertise in USFDA-compliant OSD processes like compression, granulation, and HPLC analysis.
- Strong documentation, QMS, audit, and compliance skills.
- Proficiency in GLP, GC, vendor qualification, and team leadership.
- Knowledge of routine analysis, AMV/AMD, and boiler operations.
- IT skills for software implementation like PharmaCloud.
- Excellent organizational abilities for regulated manufacturing.
Key Responsibilities
- Execute compression, granulation, and packing operations ensuring USFDA compliance.
- Perform HPLC testing, routine analysis, and documentation in QC/ADL.
- Oversee QA audits, vendor qualifications, QMS, and training programs.
- Lead production sections, manage teams, and handle GLP compliance.
- Operate boilers and maintain engineering standards.
- Support IT digitalization drives with software implementation.
Benefits/Perks
- Competitive salary with immediate joining opportunities.
- Career growth in a USFDA and UK MHRA accredited OSD facility.
- Innovative work culture focused on quality and regulated markets.
- Professional development in a sustainable, GMP-compliant environment.
- Contribution to global pharmaceutical formulations.
How to Apply
Walk-in with latest resume, CTC break-up, photo, and salary slips. If unable to attend, email resumes to aarti.oberoi@stallionlabs.com or jagdish.mathukiya@stallionlabs.com. Contact +91 9998836672 or +91 9409309964 for queries. Visit STALLion Labs for more details.
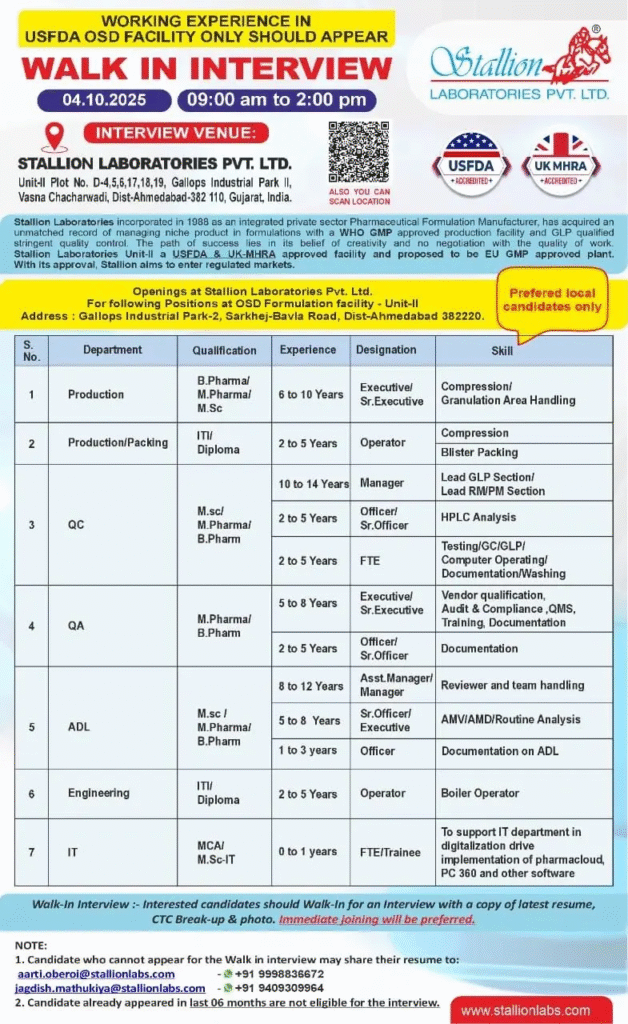
Walk-in Interview Details
- Date: October 4, 2025
- Time: 09:00 AM to 2:00 PM
- Venue: STALLion Laboratories Pvt. Ltd., Unit-II, Plot No. D-4,5,6,17,18,19, Gallops Industrial Park II, Vasna Chacharwadi, Dist-Ahmedabad-382 110, Gujarat, India
- Contact/Email: aarti.oberoi@stallionlabs.com, +91 9998836672; jagdish.mathukiya@stallionlabs.com, +91 9409309964
Why You Should Join
STALLion Laboratories offers rewarding pharmaceutical careers in a USFDA-approved OSD facility, blending innovation with stringent quality standards. As part of a growing leader in formulations, you’ll contribute to regulated market expansions, collaborate in a creative environment, and advance professionally.
With UK MHRA accreditation and EU GMP pursuits, STALLion ensures stability and global impact. Explore more QA jobs, QC jobs, and production jobs at STALLion Labs.
FAQs
Q: What experience is required for these pharma jobs at STALLion?
A: 0-14 years role-specific, but working experience in USFDA OSD facilities is mandatory.
Q: Where is the walk-in interview for pharmaceutical careers?
A: Gallops Industrial Park II, Vasna Chacharwadi, Ahmedabad, Gujarat, on October 4, 2025.
Q: What qualifications are needed for production or QA roles?
A: B.Pharm, M.Pharm, M.Sc, ITI/Diploma; USFDA OSD experience essential.
Q: How can I apply if I miss the walk-in?
A: Email your resume to aarti.oberoi@stallionlabs.com or jagdish.mathukiya@stallionlabs.com; candidates from last 6 months ineligible.
