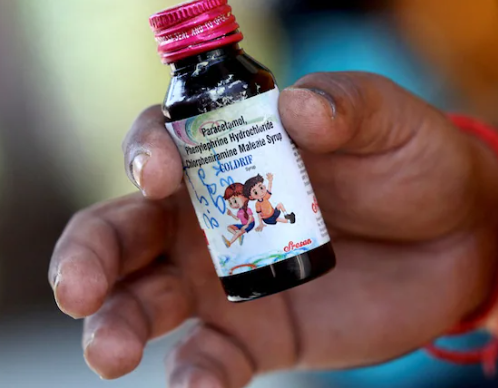
The Enforcement Directorate (ED) has launched extensive searches across Tamil Nadu following the arrest of G. Ranganathan, proprietor of Sresan Pharmaceuticals, in connection with the tragic deaths of 22 children in Madhya Pradesh linked to contaminated cough syrup.
Contents
ED Raids Multiple Locations in Tamil Nadu
According to official sources, ED officers carried out searches at seven locations across the State, targeting properties linked to Sresan Pharma employees and officials from the Tamil Nadu Drugs Control Department. The raids form part of an ongoing investigation under the Prevention of Money Laundering Act (PMLA), following the discovery of toxic contamination in the company’s Coldrif cough syrup.
Searches were also conducted at the residence of P.U. Karthikeyan, former Joint Director of Drugs Control, Food and Drug Administration. Karthikeyan had previously faced action from the Directorate of Vigilance and Anti-Corruption (DVAC) in a bribery case involving a ₹25,000 payment connected to permit processing for a soap factory.
Arrest and Charges Against Sresan Pharma Owner
The investigation intensified after G. Ranganathan, 75, was arrested last week by a Special Investigation Team (SIT) from Madhya Pradesh in Kodambakkam, Chennai. Two of his staff members were also taken into custody.
Ranganathan has been charged with:
- Culpable homicide not amounting to murder
- Drug adulteration
- Violations of the Drugs and Cosmetics Act
Toxic Tests and License Suspension
Tests on Batch SR-13 of Coldrif syrup, conducted by Tamil Nadu’s drug testing laboratory, revealed dangerous levels of diethylene glycol (DEG) — a highly toxic compound known to cause kidney and nervous system failure when ingested. Following the results, the Tamil Nadu Health Department revoked the firm’s manufacturing licence and sealed its Sunguvarchatram facility in Kancheepuram.
Officials Suspended Over Regulatory Failures
In the wake of the scandal, two senior drug inspectors — Deepa Joseph and K. Karthikeyan — were suspended for failing to adequately inspect Sresan Pharma’s manufacturing site as mandated under the law.
The Health Ministry has since announced risk-based inspections of drug manufacturing firms nationwide to prevent similar tragedies and enforce stricter adherence to quality and safety protocols.


