Explore top pharma jobs in biotechnology! Provis Biolabs recruits for QC, QA, and production positions in Hyderabad. Kickstart your pharmaceutical careers in India with innovative biologics roles.
Contents
About the Company
Founded in 2019, Provis Biolabs is a dynamic biotechnology firm specializing in premium bioreagents and biologics for global pharma needs. As a CDMO, it offers end-to-end R&D to GMP manufacturing, focusing on animal origin-free products like L-Asparaginase and Recombinant Human Albumin.
Certified with WHO-GMP, GLP, and ISO 9001:2015, Provis drives innovation in fermentation tech, serving 30+ countries with a growing pipeline of 10+ commercialized items and recent U.S. expansion for regulatory excellence and sustainable growth.
Job Details
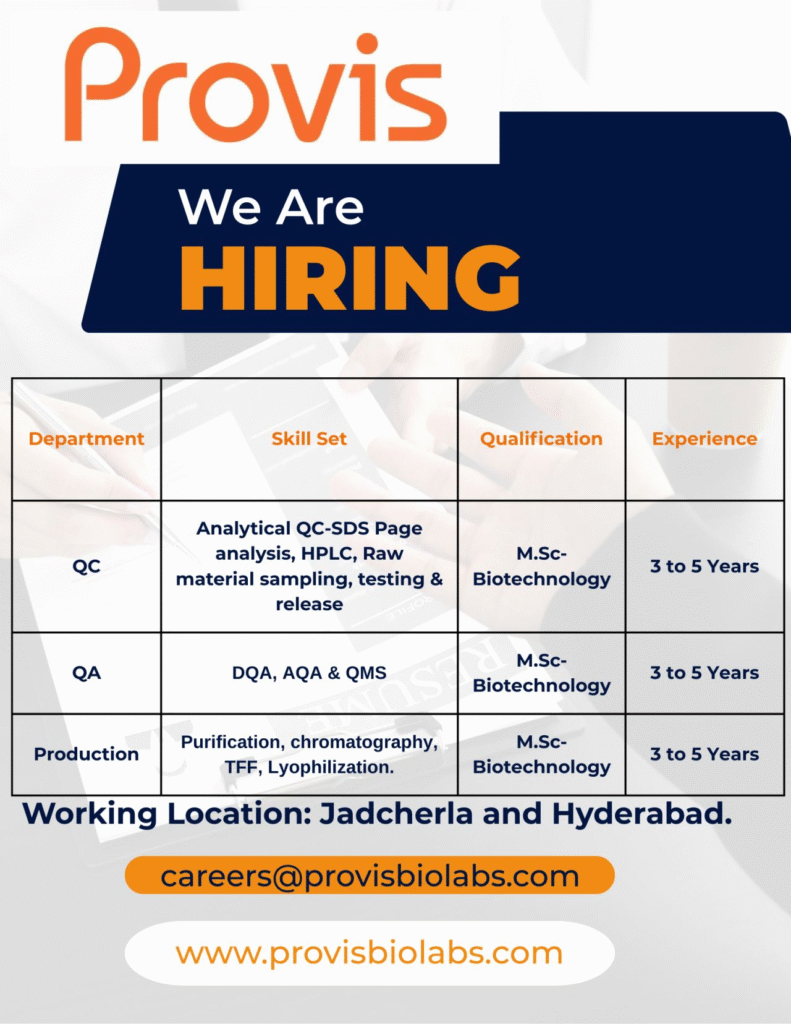
- Company Name: Provis Biolabs
- Experience: 3 to 5 years
- Qualification: M.Sc in Biotechnology
- Location: Jadcherla and Hyderabad
- Work Type: On-site
Job Description
Provis Biolabs is expanding its biopharma operations in Jadcherla and Hyderabad, hiring skilled biotechnologists for QC, QA, and Production teams. These roles support cutting-edge biologics manufacturing, from testing to purification, in a GMP-compliant environment. Join a collaborative group advancing therapeutic innovations for global markets.
QC Analyst
- Department: Quality Control (QC)
- Role: Perform analytical testing and raw material release
- Experience: 3 to 5 years
- Education/Qualification: M.Sc in Biotechnology
QA Specialist
- Department: Quality Assurance (QA) – DQA, AQA & QMS
- Role: Ensure compliance in documentation, audits, and quality management systems
- Experience: 3 to 5 years
- Education/Qualification: M.Sc in Biotechnology
Production Technician
- Department: Production
- Role: Execute purification and downstream processing for biologics
- Experience: 3 to 5 years
- Education/Qualification: M.Sc in Biotechnology
Skills/Qualifications
- Expertise in SDS-PAGE analysis, HPLC, and raw material sampling/testing
- Proficiency in GMP compliance, DQA, AQA, and QMS documentation
- Hands-on experience with purification, chromatography, TFF, and lyophilization
- Strong analytical skills for biotech product release and validation
- Knowledge of regulatory standards like WHO-GMP and ISO
- Excellent attention to detail and problem-solving in lab settings
- Team collaboration for cross-functional biopharma projects
- Commitment to safety and ethical sourcing in animal origin-free processes
Key Responsibilities
- Conduct HPLC and SDS-PAGE for QC analysis
- Sample and test raw materials for release
- Manage QMS documentation and audits
- Perform DQA and AQA compliance checks
- Execute chromatography and TFF purification
- Operate lyophilization for product stability
- Ensure GMP adherence in all processes
- Support batch release and deviation handling
Benefits/Perks
- Career growth with training in advanced biotech techniques
- Learning opportunities in CDMO operations and global projects
- Competitive salary and performance incentives
- Collaborative work culture fostering innovation
- Global exposure to international pharma partnerships
- Health insurance and wellness programs
- On-site facilities for work-life balance
- ESG-focused initiatives for sustainable impact
How to Apply
Submit your resume, cover letter, and qualifications to careers@provisbiolabs.com, referencing the desired role. Visit www.provisbiolabs.com for more details. For tailored pharma jobs advice, check Pharma Recruiter.
Verified Post
The post is released by the Provis LinkedIn page. Click here to visit the post
Launch your biotech career—apply today and contribute to groundbreaking bioreagents!
Why You Should Join
Provis Biolabs cultivates a culture of excellence and ethical innovation, recognized for its rapid growth and WHO-GMP compliance in biologics. Enjoy long-term stability in a expanding CDMO with U.S. outreach, plus hands-on learning in fermentation and purification tech.
Thrive in an environment prioritizing safety, diversity, and patient impact, building a fulfilling path in India’s biotech hub. Explore more at Pharma Recruiter.
FAQs
What qualifications are required for Provis Biolabs’ biotech roles?
M.Sc in Biotechnology with 3-5 years of experience in QC, QA, or production. Focus on analytical and GMP skills for biologics handling.
How do I apply for QC or QA jobs at Provis?
Email your resume to careers@provisbiolabs.com with role details. Applications reviewed promptly; prepare for technical interviews on HPLC and compliance.
Where are the working locations for these positions?
Jadcherla and Hyderabad facilities, offering on-site roles in state-of-the-art biotech labs for hands-on manufacturing.
What growth opportunities exist at Provis Biolabs?
Progress to senior QA/QC leads or production management, with training in global CDMOs and pipeline expansions for sustained pharma career advancement.



Is there vacancies for freshers