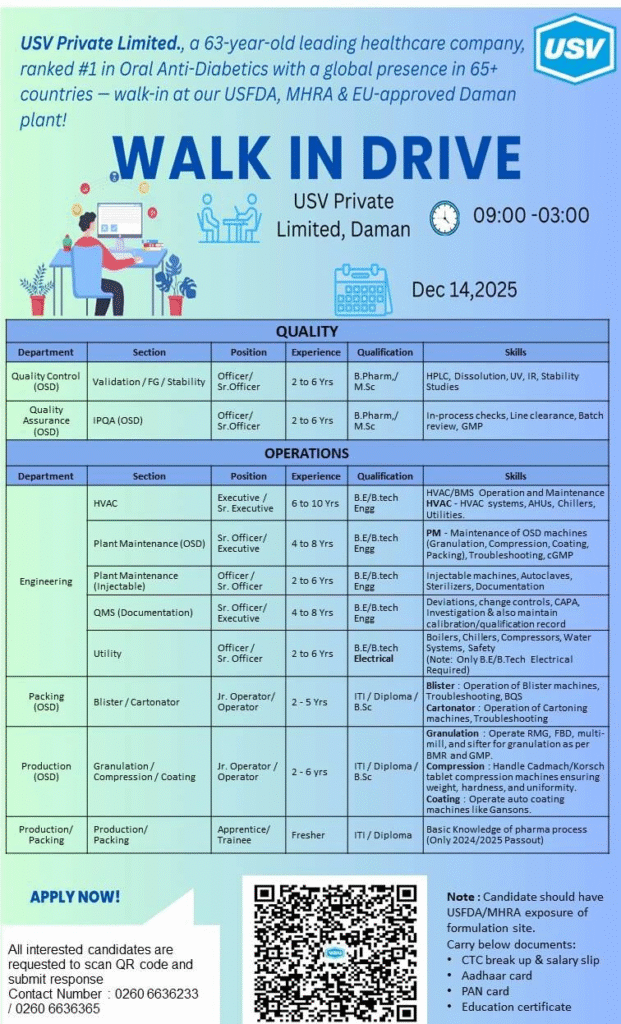
Discover top pharma jobs in India at USV’s walk-in interview for QA, QC, production roles. Gain exposure in USFDA-approved facilities. Freshers and experienced welcome in Daman!
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Quality Control (OSD) – Validation/FG/Stability
- 3.2 Quality Assurance (OSD) – IPQA
- 3.3 Engineering – HVAC
- 3.4 Engineering – Plant Maintenance (OSD)
- 3.5 Engineering – Plant Maintenance (Injectable)
- 3.6 Engineering – QMS (Documentation)
- 3.7 Operations – Utility
- 3.8 Packing (OSD) – Blister/Cartonator
- 3.9 Production (OSD) – Granulation/Compression/Coating
- 3.10 Production/Packing – Apprentice/Trainee
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
USV Private Limited, founded in 1961 by Dr. Vithal Balkrishna Gandhi, stands as a 63-year-old pioneer in healthcare innovation. Ranked #1 in Oral Anti-Diabetics, it leads in diabetes and cardiology solutions with a diverse generics portfolio.
Operating in 75+ countries and generating nearly $500 million in annual revenue, USV ensures regulatory excellence through USFDA, MHRA, and EU-approved plants, driving global growth and sustainable pharma careers in India.
Job Details
- Company Name: USV Private Limited
- Experience: Varies by role (Fresher to 10 years)
- Qualification: B.Pharm/M.Sc/B.E/B.Tech (Engineering)/ITI/Diploma/B.Sc
- Location: Daman Plant
- Work Type: On-site
Job Description
USV Private Limited invites pharma professionals for a dynamic walk-in drive at its state-of-the-art Daman plant, focusing on quality, operations, engineering, and production.
These roles offer hands-on experience in GMP-compliant environments, ideal for advancing pharmaceutical careers in India. With a preference for USFDA/MHRA exposure, positions span OSD, injectables, and utilities.
Quality Control (OSD) – Validation/FG/Stability
- Department: Quality – Validation/FG/Stability
- Market: Global healthcare and pharmaceuticals
- Role: Officer/Sr. Officer ensuring product stability and validation
- Experience: 2 to 6 years
- Education/Qualification: B.Pharm/M.Sc
Quality Assurance (OSD) – IPQA
- Department: Quality Assurance (OSD)
- Market: Global healthcare and pharmaceuticals
- Role: Officer/Sr. Officer for in-process quality checks
- Experience: 2 to 6 years
- Education/Qualification: B.Pharm/M.Sc
Engineering – HVAC
- Department: Operations – HVAC
- Market: Global healthcare and pharmaceuticals
- Role: Executive/Sr. Executive managing HVAC systems
- Experience: 6 to 10 years
- Education/Qualification: B.E/B.Tech Engineering
Engineering – Plant Maintenance (OSD)
- Department: Engineering – Plant Maintenance (OSD)
- Market: Global healthcare and pharmaceuticals
- Role: Sr. Officer/Executive for OSD machine upkeep
- Experience: 4 to 8 years
- Education/Qualification: B.E/B.Tech Engineering
Engineering – Plant Maintenance (Injectable)
- Department: Engineering – Plant Maintenance (Injectable)
- Market: Global healthcare and pharmaceuticals
- Role: Officer/Sr. Officer for injectable equipment
- Experience: 2 to 6 years
- Education/Qualification: B.E/B.Tech Engineering
Engineering – QMS (Documentation)
- Department: Engineering – QMS (Documentation)
- Market: Global healthcare and pharmaceuticals
- Role: Sr. Officer/Executive handling compliance records
- Experience: 4 to 8 years
- Education/Qualification: B.E/B.Tech Engineering
Operations – Utility
- Department: Operations – Utility
- Market: Global healthcare and pharmaceuticals
- Role: Officer/Sr. Officer for utility systems
- Experience: 2 to 6 years
- Education/Qualification: B.E/B.Tech Electrical
Packing (OSD) – Blister/Cartonator
- Department: Packing (OSD)
- Market: Global healthcare and pharmaceuticals
- Role: Jr. Operator/Operator for blister and cartoning
- Experience: 2 to 5 years
- Education/Qualification: ITI/Diploma/B.Sc
Production (OSD) – Granulation/Compression/Coating
- Department: Production (OSD)
- Market: Global healthcare and pharmaceuticals
- Role: Jr. Operator/Operator for granulation processes
- Experience: 2 to 6 years
- Education/Qualification: B.Sc/ITI/Diploma
Production/Packing – Apprentice/Trainee
- Department: Production/Packing
- Market: Global healthcare and pharmaceuticals
- Role: Apprentice/Trainee in pharma processes
- Experience: Fresher
- Education/Qualification: ITI/Diploma (2024/2025 passouts)
Skills/Qualifications
- Proficiency in HPLC, Dissolution, UV, IR, and Stability Studies for QC roles
- Expertise in in-process checks, line clearance, batch review, and GMP compliance
- Knowledge of HVAC/BMS operations, chillers, AHUs, and utilities maintenance
- Hands-on experience with OSD/injectable machines, troubleshooting, and cGMP
- Skills in deviations, CAPA, change controls, and calibration documentation
- Operation of boilers, compressors, water systems; electrical engineering basics
- Familiarity with blister/cartonator machines, granulation tools like RMG/FBD
- Basic pharma process knowledge; USFDA/MHRA exposure preferred
- Strong analytical, problem-solving, and documentation abilities
Key Responsibilities
- Perform HPLC analysis and stability testing daily
- Conduct IPQA checks during OSD production runs
- Maintain HVAC systems and troubleshoot AHUs
- Oversee granulation and compression machine upkeep
- Document deviations and implement CAPA actions
- Operate utility equipment like boilers safely
- Run blister packing lines with quality assurance
- Assist in coating processes per BMR guidelines
- Support packing operations for efficiency
- Learn GMP basics as a trainee apprentice
Benefits/Perks
- Competitive salary with performance incentives
- Career growth in global pharma leadership roles
- Hands-on training in USFDA-compliant facilities
- Collaborative work culture fostering innovation
- Opportunities for international exposure
- Health insurance and employee wellness programs
How to Apply
All interested candidates should attend the walk-in drive with required documents: CTC breakup & salary slips, Aadhaar card, PAN card, and education certificates. For additional pharma job resources, visit Pharma Recruiter.
Scan the QR code in the original posting or contact 0260 6636233 / 0260 6636365 for queries. Secure your spot in India’s top pharmaceutical careers—apply now!
Walk-in Interview Details
- Date: December 14, 2025
- Time: 09:00 AM to 03:00 PM
- Venue: USV Private Limited, Daman Plant
- Contact/Email: 0260 6636233 / 0260 6636365
Why You Should Join
USV Private Limited cultivates a culture of excellence and employee recognition, where innovation meets regulatory rigor in diabetes and cardiology solutions. With stable long-term careers in a firm expanding across 75 countries, you’ll access unmatched learning through advanced training and global projects.
Embrace a compliance-driven environment that propels QA jobs, production roles, and engineering expertise forward in India’s thriving pharma sector.
FAQs
Who qualifies for USV’s walk-in pharma jobs?
Candidates with B.Pharm/M.Sc for quality roles, B.E/B.Tech for engineering, and ITI/Diploma/B.Sc for operations/production. USFDA/MHRA exposure preferred; freshers for trainee positions.
What’s the process for this walk-in interview?
Attend on Dec 14, 2025, at Daman plant with documents. Direct interviews; no prior registration needed. Expect on-site assessments for QA, QC, and production jobs.
Are there salary details for these positions?
Salaries vary by experience (2-10 years) and role, competitive for pharma standards. Discuss during interview; CTC breakup required for experienced candidates.
What growth opportunities exist at USV?
Rapid advancement from operator to executive levels, with global exposure and skill-building in GMP-compliant plants. Top performers transition to leadership in India’s #1 anti-diabetics firm.


