Join Rusan Pharma walk-in interview in Ankleshwar for API production jobs, QC jobs, QA jobs. Experienced professionals needed on 28 Dec 2025 for pharmaceutical careers in India.
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Officer / Jr. Officer (GMP Co-ordinator) – Production
- 3.2 Jr. Officer / Officer – Quality Control
- 3.3 Executive / Sr. Officer – Analytical Quality Assurance
- 3.4 Executive (Shift Incharge) – Production
- 3.5 Sr. Officer / Officer / Jr. Officer – Quality Assurance
- 3.6 Assistant Manager – Quality Assurance
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
Rusan Pharma Ltd. is a fully integrated global pharmaceutical company specializing in addiction treatment and pain management. It offers innovative, one-stop solutions through APIs, formulations, and advanced therapeutics.
Technology-driven and managed by professional technocrats, Rusan maintains high standards of quality, regulatory compliance, and global reach. The company focuses on critical healthcare areas, delivering impactful solutions worldwide.
Job Details
- Company Name: Rusan Pharma Ltd.
- Experience: 1–9 years (varies by role)
- Qualification: B.Sc / M.Sc
- Location: Ankleshwar, Gujarat
- Work Type: On-site
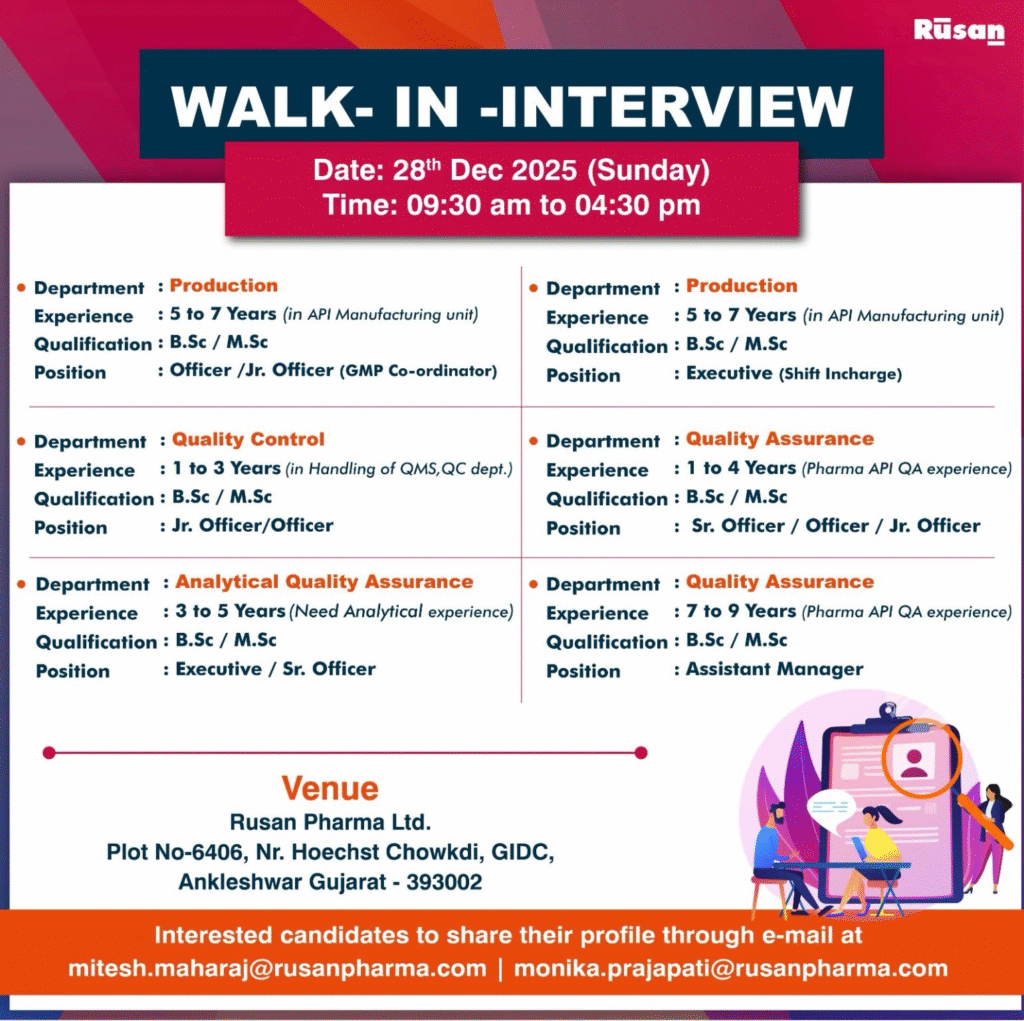
Job Description
Rusan Pharma Ltd. is conducting a walk-in interview on 28 December 2025 for experienced professionals in its API manufacturing unit at Ankleshwar. These roles span Production, Quality Control, Analytical Quality Assurance, and Quality Assurance, requiring hands-on API pharma experience.
Officer / Jr. Officer (GMP Co-ordinator) – Production
- Department: Production
- Role: GMP Co-ordinator
- Experience: 5-7 years in API Manufacturing
- Education/Qualification: B.Sc / M.Sc
Jr. Officer / Officer – Quality Control
- Department: Quality Control
- Role: Handling QMS in QC Department
- Experience: 1-3 years
- Education/Qualification: B.Sc / M.Sc
Executive / Sr. Officer – Analytical Quality Assurance
- Department: Analytical Quality Assurance
- Role: Analytical Experience Required
- Experience: 3-5 years
- Education/Qualification: B.Sc / M.Sc
Executive (Shift Incharge) – Production
- Department: Production
- Role: Shift Incharge
- Experience: 5-7 years in API Manufacturing
- Education/Qualification: B.Sc / M.Sc
Sr. Officer / Officer / Jr. Officer – Quality Assurance
- Department: Quality Assurance
- Role: Pharma API QA Experience
- Experience: 1-4 years
- Education/Qualification: B.Sc / M.Sc
Assistant Manager – Quality Assurance
- Department: Quality Assurance
- Role: Pharma API QA Experience
- Experience: 7-9 years
- Education/Qualification: B.Sc / M.Sc
Skills/Qualifications
- B.Sc or M.Sc in relevant scientific discipline
- Mandatory experience in API pharmaceutical manufacturing
- Knowledge of GMP coordination and compliance
- Expertise in Quality Management Systems (QMS) for QC roles
- Strong analytical skills for Analytical QA positions
- Proven QA experience in pharma API environment
- Ability to manage shifts and ensure operational efficiency
Key Responsibilities
- Coordinate GMP activities and documentation
- Handle QMS processes and compliance checks
- Perform analytical testing and validation
- Manage production shifts effectively
- Oversee quality assurance audits and reviews
- Ensure adherence to regulatory standards
Benefits/Perks
- Competitive salary in specialty pharma sector
- Opportunity to work in addiction and pain management therapeutics
- Career growth in a global, innovative company
- Exposure to advanced API manufacturing
- Stable environment with focus on quality
- Professional development in critical healthcare areas
How to Apply
Attend the walk-in interview with your updated resume and documents. Alternatively, email your profile to mitesh.maharaj@rusanpharma.com or monika.prajapati@rusanpharma.com.
For more pharma walk-in opportunities, visit Pharma Recruiter. Take your next career step with Rusan—attend on 28 Dec 2025!
Walk-in Interview Details
- Date: 28th December 2025 (Sunday)
- Time: 09:30 am to 04:30 pm
- Venue: Rusan Pharma Ltd., Plot No-6406, Nr. Hoechst Chowkdi, GIDC, Ankleshwar, Gujarat – 393002
Why You Should Join
Rusan Pharma provides a meaningful career in a specialized global company focused on addiction treatment and pain management. Employees contribute to innovative, life-changing therapeutics in a technology-driven, compliant environment.
With opportunities for professional growth, exposure to international standards, and long-term stability, Rusan offers a rewarding path in the pharmaceutical industry.
FAQs
What experience is required for Rusan Pharma roles?
Experience ranges from 1-9 years in API pharma, specific to departments like GMP, QMS, analytical, and QA.
How can I apply for the walk-in interview?
Attend directly on 28 Dec 2025 from 9:30 am–4:30 pm, or email your resume to the provided contacts.
Is API manufacturing experience mandatory?
Yes, relevant API pharma experience is required for all positions as per the specified years.
What qualifications are needed?
B.Sc or M.Sc qualification is essential for all listed roles in Production, QC, and QA.


