Discover rewarding pharma jobs in Pune at Lupin! Exciting openings for Trainee Officer in Production Mammalian, Research Associate in Biotech R&D, and Quality Assurance Officer. Join a global pharmaceutical leader today.
Contents
About the Company
Lupin Limited, founded in 1968, is a leading Indian multinational pharmaceutical company headquartered in Mumbai. Renowned for generic drugs, branded formulations, active pharmaceutical ingredients (APIs), and biotechnology products, Lupin operates in over 100 countries. The company drives innovation, maintains stringent regulatory compliance, and delivers high-quality, affordable healthcare solutions worldwide.
Job Details
- Company Name: Lupin Limited
- Experience: 1-4 years (varies by role; freshers eligible for select positions)
- Qualification: Graduation or Master’s/Post Graduation in Biotechnology
- Location: Pune, Maharashtra, India
- Work Type: On-site
Job Description
Lupin strengthens its biotechnology and pharmaceutical operations in Pune with multiple openings across production, research & development, and quality assurance.
These roles deliver hands-on experience in advanced bioprocesses, analytical development, and GMP-compliant manufacturing. Professionals contribute to innovative, high-impact projects in a regulated environment.
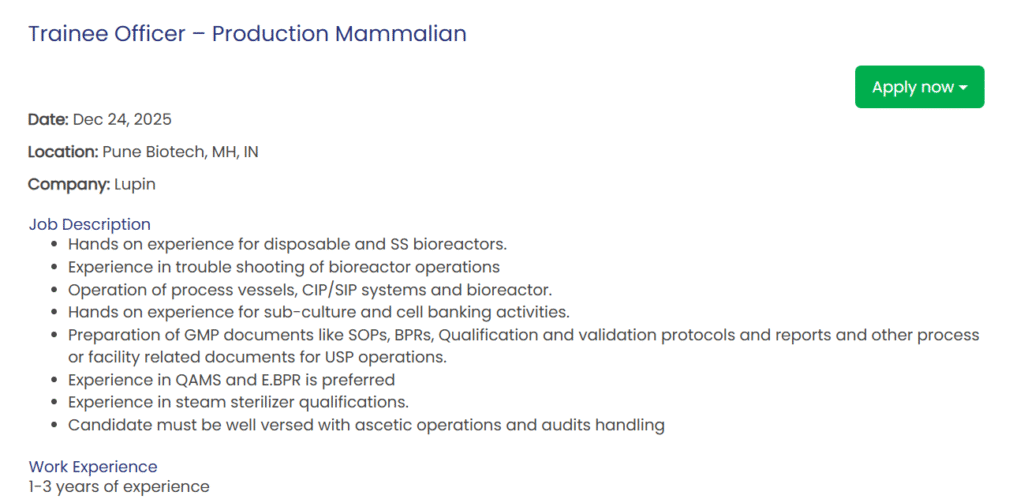
Trainee Officer – Production Mammalian
- Location: Pune Biotech, MH, IN
- Experience: 1-3 years
- Education/Qualification: Graduation in Biotechnology
Key Responsibilities:
- Operate disposable and stainless steel (SS) bioreactors with hands-on expertise.
- Troubleshoot bioreactor operations effectively.
- Manage process vessels, CIP/SIP systems, and bioreactors.
- Perform sub-culture and cell banking activities.
- Prepare GMP documents including SOPs, BPRs, qualification/validation protocols, and reports for USP operations.
- Handle QAMS and E.BPR (preferred).
- Conduct steam sterilizer qualifications.
- Ensure proficiency in aseptic operations and audit handling.
Research Associate
- Location: Pune_RD, MH, IN
- Experience: Freshers and experienced candidates welcome
- Education/Qualification: Degree in Biotechnology or related field implied
Key Responsibilities:
- Develop and qualify analytical methods.
- Execute method transfers to Quality Control (QC).
- Conduct routine analytical testing to support process teams via in-process sample analysis.
- Maintain documentation with full regulatory compliance.
Officer: Quality Assurance
- Location: Pune Biotech, MH, IN
- Experience: 1-4 years
- Education/Qualification: Master’s or Post Graduation in Biotechnology
Key Responsibilities:
- Inspect manufacturing and warehouse facilities to enforce GMP compliance and approved procedures.
- Provide line clearance for manufacturing processes.
- Maintain GMP-ready facilities and follow up on findings closure.
- Identify and implement new procedures with the manufacturing team; perform gap analysis.
- Review and initiate QMS elements (change control, deviation, CAPA, market complaints, investigations).
- Prepare and review Annual Product Quality Review (APQR) for manufactured products.
Skills/Qualifications
- Hands-on experience with bioreactors, CIP/SIP, and aseptic techniques (Production role).
- Knowledge of analytical method development, qualification, and transfers (R&D role).
- Expertise in GMP compliance, QMS elements, audits, and documentation (QA role).
- Proficiency in GMP documentation (SOPs, BPRs, protocols/reports).
- Strong understanding of regulatory requirements and aseptic operations.
- Soft skills: Strategic Agility, Innovation & Creativity, Customer Centricity, Collaboration, Process Excellence, Result Orientation.
Key Responsibilities
- Execute upstream/downstream bioprocessing or analytical testing with precision.
- Ensure compliance with GMP and regulatory standards across operations.
- Troubleshoot processes and handle deviations/investigations.
- Prepare and review critical documentation for audits and validations.
- Support manufacturing through line clearance, facility maintenance, and quality oversight.
- Drive continuous improvement via CAPA and procedure updates.
Benefits/Perks
- Competitive salary and comprehensive benefits package.
- Excellent career growth in a global pharmaceutical company.
- Continuous learning and professional development opportunities.
- Innovative, collaborative, and supportive work culture.
- Exposure to cutting-edge biotechnology and global projects.
How to Apply
Apply online through the official Lupin careers portal at https://careers.lupin.com/. Search for positions in Pune Biotech or R&D and submit your updated resume.
Verified Post
Verification: To confirm the legitimacy of this posting, you can view the original announcement on the Lupin Career page.


Take the next step in your pharmaceutical career with Lupin—apply today and contribute to global healthcare innovation!
For more pharma job opportunities across India, visit Pharma Recruiter.
Why You Should Join
Lupin offers a dynamic culture that values innovation, integrity, and employee growth. Professionals enjoy long-term career stability, world-class training, and the chance to work on life-changing therapies. Join a recognized global leader committed to quality, compliance, and employee success.
FAQs
What qualifications are required for these Lupin Pune roles?
Candidates need a Graduation or Master’s in Biotechnology, with 1-4 years of relevant experience (freshers eligible for Research Associate).
How can I apply for these pharmaceutical jobs at Lupin?
Submit applications online via the official portal: https://careers.lupin.com/. No walk-in interviews are scheduled.
Are these roles suitable for freshers?
Yes, the Research Associate position welcomes biotechnology freshers; other roles prefer 1-4 years of experience.
What career growth can I expect at Lupin?
Lupin provides strong progression opportunities, global exposure, training programs, and a focus on innovation and talent development.


