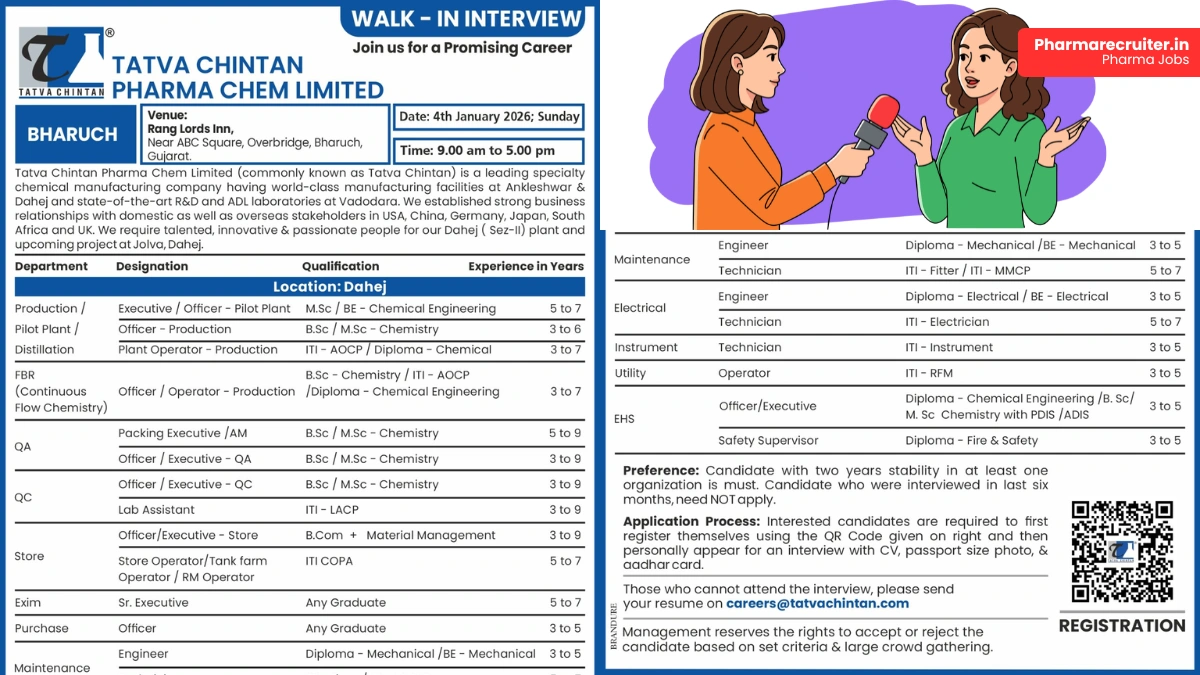
Join Tatva Chintan Pharma Chem Limited for exciting pharmaceutical careers in India. Attend the walk-in interview on 4th January 2026 for production jobs, QA jobs, QC jobs, maintenance, and EHS roles at the new Dahej plant.
Contents
About the Company
Tatva Chintan Pharma Chem Limited is a leading specialty chemical manufacturing company with world-class facilities at Ankleshwar and Dahej, along with state-of-the-art R&D and ADL laboratories in Vadodara.
The company has built strong business relationships with stakeholders in the USA, China, Germany, Japan, South Africa, and the UK.
Focused on innovation and quality, Tatva Chintan is expanding its operations with an upcoming project at Jolva, Dahej SEZ-II, and is seeking passionate professionals to join its growth journey.
Job Details
- Company Name: Tatva Chintan Pharma Chem Limited
- Location: Dahej SEZ-II, Gujarat (Upcoming project at Jolva)
- Experience: Varies by role (3–9 years)
- Qualification: B.Sc/M.Sc Chemistry, BE/Diploma Chemical/Mechanical/Electrical Engineering, ITI (AOCP, Fitter, Electrician, COPA, LACP, RFM, Instrument), B.Com, Any Graduate, Diploma Fire & Safety, PDIS/ADIS
- Work Type: On-site
Job Description
Tatva Chintan Pharma Chem Limited is expanding its Dahej SEZ-II facility and is hiring experienced professionals across multiple departments for its upcoming Jolva project.
These full-time, on-site roles offer opportunities to work in a modern specialty chemical and pharmaceutical intermediate manufacturing environment.
Production / Pilot Plant / Distillation / FBR Roles
Executive / Officer – Pilot Plant
- Qualification: M.Sc / BE Chemical Engineering
- Experience: 5–7 years
Officer – Production
- Qualification: B.Sc / M.Sc Chemistry
- Experience: 3–6 years
Plant Operator – Production
- Qualification: ITI-AOCP / Diploma Chemical
- Experience: 3–7 years
FBR Officer / Operator (Continuous Flow Chemistry)
- Qualification: B.Sc Chemistry / ITI-AOCP / Diploma Chemical Engineering
- Experience: 3–7 years
Packing Executive / Assistant Manager
- Qualification: B.Sc / M.Sc Chemistry
- Experience: 5–9 years
Quality Assurance & Quality Control Roles
Officer / Executive – QA
- Qualification: B.Sc / M.Sc Chemistry
- Experience: 3–9 years
Officer / Executive – QC
- Qualification: B.Sc / M.Sc Chemistry
- Experience: 3–9 years
Lab Assistant – QC
- Qualification: ITI-LACP
- Experience: 3–9 years
Store & Materials Roles
Officer / Executive – Store
- Qualification: B.Com / Material Management
- Experience: 3–9 years
Store Operator / Tank Farm Operator / RM Operator
- Qualification: ITI COPA
- Experience: 5–7 years
Exim & Purchase Roles
Senior Executive – Exim
- Qualification: Any Graduate
- Experience: 5–7 years
Officer – Purchase
- Qualification: Any Graduate
- Experience: 3–5 years
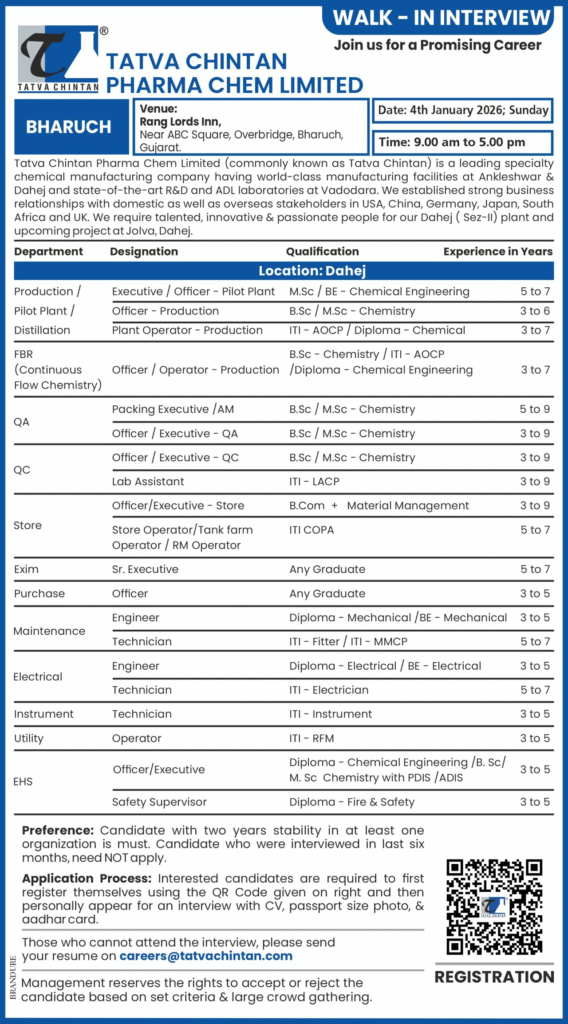
Maintenance & Engineering Roles
Engineer – Maintenance
- Qualification: Diploma / BE Mechanical
- Experience: 3–5 years
Technician – Maintenance
- Qualification: ITI Fitter / MMCP
- Experience: 5–7 years
Engineer / Technician – Electrical
- Qualification: Diploma / BE Electrical
- Experience: 3–5 years
Technician – Instrument
- Qualification: ITI Instrument / Electrician
- Experience: 3–7 years
Utility & EHS Roles
Operator – Utility
- Qualification: ITI-RFM
- Experience: 3–5 years
Officer / Executive – EHS
- Qualification: Diploma Chemical Engineering / B.Sc / M.Sc Chemistry with PDIS / ADIS
- Experience: 3–5 years
Safety Supervisor
- Qualification: Diploma Fire & Safety
- Experience: 3–5 years
Skills/Qualifications
- Relevant technical qualification matching the role
- 3–9 years of experience in pharmaceutical or specialty chemical manufacturing
- At least 2 years of job stability in one previous organization (preferred)
- Hands-on knowledge of production, quality, maintenance, or safety processes
- Familiarity with GMP, safety standards, and regulatory requirements
Key Responsibilities
- Operate and monitor production and pilot plant processes
- Conduct quality assurance and control analysis
- Perform equipment maintenance and troubleshooting
- Ensure adherence to EHS and safety guidelines
- Handle documentation, materials, and logistics efficiently
- Support export-import and procurement activities
Benefits/Perks
- Competitive salary and benefits
- Clear career growth path in a growing organization
- Exposure to international clients and advanced technologies
- Continuous learning and skill development
- Stable and professional work environment
How to Apply
Candidates must first register using the QR code in the official advertisement. Then attend the walk-in interview with your updated CV, passport-size photograph, and Aadhar card. If unable to attend, email your resume to careers@tatvachintan.com.
Verified Post
Verification: To confirm the legitimacy of this posting, you can view the original announcement on the Tatva Chintan Pharma Chem Limited LinkedIn page.
Explore more pharma opportunities at Pharma Recruiter. Don’t miss this chance – register and apply today!
Walk-in Interview Details
- Date: 4th January 2026 (Sunday)
- Time: 9:00 am to 5:00 pm
- Venue: Rang Lords Inn, Near ABC Square, Overbridge, Bharuch, Gujarat
Why You Should Join
Tatva Chintan offers a professional environment that values innovation, quality, and long-term employee growth.
With global clientele and modern facilities, you’ll gain valuable experience in specialty chemicals while enjoying job stability and opportunities to advance in a respected, expanding company.
FAQs
Who is eligible to apply?
Candidates with required qualifications, 3–9 years experience, and preferably 2 years stability in at least one organization. Candidates interviewed in the last 6 months need not apply.
Can I apply if I cannot attend the walk-in?
Yes, email your resume to careers@tatvachintan.com for consideration.
What documents are required for the interview?
Updated CV, passport-size photo, Aadhar card, and prior QR code registration.
What growth opportunities are available?
Tatva Chintan provides strong career progression, international exposure, and professional development in a fast-growing specialty chemical company.