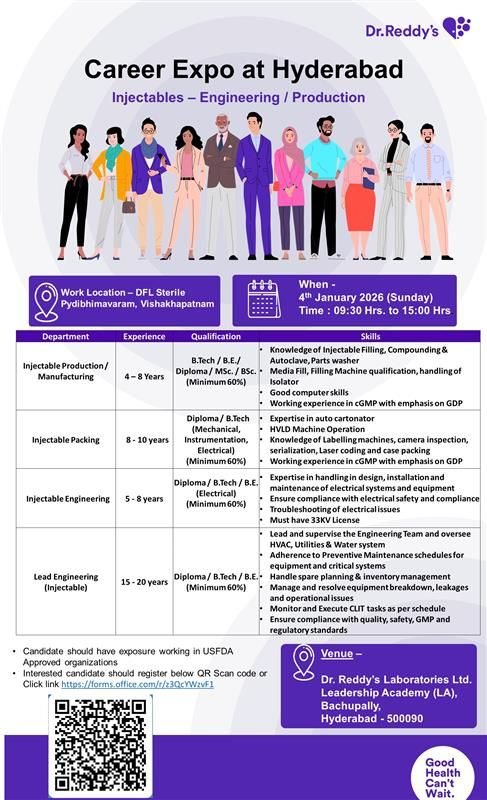
Join Dr. Reddy’s Laboratories Career Expo in Hyderabad on 4th January 2026. Exciting opportunities in sterile injectables production, packing, and engineering roles at Visakhapatnam facility for experienced pharma professionals.
Contents
About the Company
Dr. Reddy’s Laboratories Ltd. is a leading global pharmaceutical company headquartered in Hyderabad, India. Founded in 1984, it specializes in affordable generics, biosimilars, and innovative medicines across oncology, gastroenterology, cardiology, and pain management.
With USFDA-approved manufacturing facilities and presence in over 60 countries, Dr. Reddy’s emphasizes quality, compliance, and innovation to deliver good health worldwide.
Job Details
- Company Name: Dr. Reddy’s Laboratories Ltd.
- Location: DFL Sterile, Pydibhimavaram, Visakhapatnam, Andhra Pradesh
- Experience: Varies by role (4–20 years)
- Qualification: B.Tech/B.E./Diploma/M.Sc/B.Sc (Minimum 60% marks); Specific streams in Mechanical, Instrumentation, Electrical for engineering roles
- Work Type: On-site
Job Description
Dr. Reddy’s Laboratories is hosting a Career Expo to hire experienced professionals for its state-of-the-art sterile injectables facility in Visakhapatnam.
These roles demand expertise in USFDA-approved environments and focus on high-quality injectable manufacturing and engineering. All positions require prior exposure to regulated pharmaceutical operations.
Injectable Production / Manufacturing
- Experience: 4–8 years
- Qualification: B.Tech/B.E./Diploma/M.Sc/B.Sc (Minimum 60% marks)
Injectable Packing
- Experience: 8–10 years
- Qualification: B.Tech/B.E./Diploma/M.Sc/B.Sc (Minimum 60% marks)
Injectable Engineering
- Experience: 5–8 years
- Qualification: Diploma/B.Tech/B.E. in Electrical (Minimum 60% marks)
Lead Engineering (Injectables)
- Experience: 15–20 years
- Qualification: Diploma/B.Tech/B.E. (Minimum 60% marks); Mechanical, Instrumentation, or Electrical preferred
Skills/Qualifications
- Exposure to USFDA-approved injectable manufacturing organizations
- Minimum 60% marks in relevant qualification
- Strong knowledge of cGMP with emphasis on GDP
- Good computer skills for documentation and systems
- Specific expertise in injectable processes (filling, compounding, autoclave, isolator, media fill)
- Proficiency in packing operations (auto cartonator, HVLD, labelling, serialization, camera inspection)
- Electrical systems design, installation, maintenance, and troubleshooting
- For senior roles: 33KV electrical license and leadership experience
Key Responsibilities
- Operate injectable filling, compounding, autoclave, and parts washer efficiently
- Perform media fill and filling machine qualifications
- Handle isolator operations and VHP processes
- Manage auto cartonator, HVLD, labelling, serialization, and case packing
- Design, install, and maintain electrical systems with safety compliance
- Lead engineering team and oversee HVAC, utilities, and water systems
- Execute preventive maintenance and spare inventory management
- Resolve equipment breakdowns and monitor CLIT tasks
- Ensure adherence to quality, safety, GMP, and regulatory standards
Benefits/Perks
- Competitive salary and benefits package
- Career progression in a globally respected pharma leader
- Exposure to advanced sterile injectables technology
- Professional development in regulated environments
- Stable and innovative work culture focused on health impact
How to Apply
Interested candidates must pre-register by scanning the QR code from the official advertisement or clicking the registration link: https://forms.office.com/r/z3QcYWzvF1.
After registration, attend the Career Expo in person with your updated CV and documents. Discover more pharma job openings at Pharma Recruiter. Register now and accelerate your career with Dr. Reddy’s!
Career Expo Details
- Date: 4th January 2026 (Sunday)
- Time: 09:30 Hrs to 15:00 Hrs
- Venue: Dr. Reddy’s Laboratories Ltd. Leadership Academy (LA), Bachupally, Hyderabad – 500090
Why You Should Join
Dr. Reddy’s Laboratories offers a purpose-driven environment where professionals contribute to life-changing medicines.
With a strong focus on compliance, innovation, and employee growth, it provides long-term stability, global exposure, and opportunities to excel in sterile injectables within USFDA-approved facilities.
FAQs
Who is eligible for these roles?
Experienced candidates (4–20 years) with relevant qualifications (minimum 60% marks) and mandatory exposure to USFDA-approved injectable manufacturing.
Is pre-registration mandatory?
Yes, all candidates must register via the provided link or QR code before attending the Career Expo.
What documents should I carry?
Updated CV, educational certificates, experience letters, registration confirmation, and valid ID proof.
Are there opportunities for career growth?
Yes, Dr. Reddy’s provides excellent progression paths, global projects, and development programs in a leading pharmaceutical organization.


