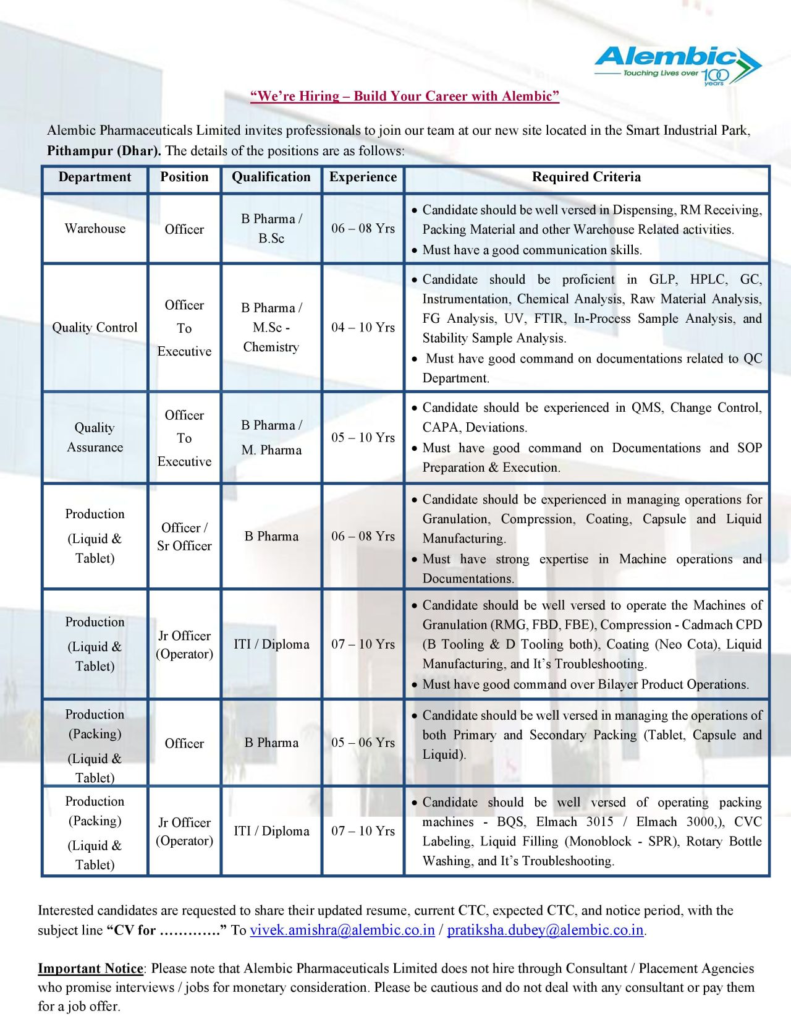
Alembic Pharmaceuticals Limited, a century-old leader in the Indian pharmaceutical industry, invites professionals to join our new state-of-the-art facility at Smart Industrial Park, Pithampur (Dhar). We are hiring for roles in Warehouse, Quality Control, Quality Assurance, and Production (Liquid & Tablet) departments. Build your career with a trusted name committed to touching lives through innovation and quality!
Contents
Event Details
Application Process: Ongoing (no walk-in specified; apply via email)
Work Location: Alembic Pharmaceuticals Ltd., Smart Industrial Park, Pithampur, Dhar, Madhya Pradesh
Apply: Email updated resume to vivek.amishra@alembic.co.in or pratiksha.dubey@alembic.co.in with subject line “CV for [Position]”
Important Notice: Alembic does not hire through consultants or agencies charging fees. Beware of fraudulent job offers.
Open Positions
We’re hiring for multiple roles across departments. Below are the details:
| Department | Position | Qualification | Experience | Key Responsibilities |
|---|---|---|---|---|
| Warehouse | Officer | B.Pharm., B.Sc. | 6-8 Years | Manage dispensing, RM receiving, packing material handling |
| Quality Control | Officer to Executive | B.Pharm., M.Sc. (Chem) | 4-10 Years | Perform GLP, HPLC, GC, chemical/stability analysis, documentation |
| Quality Assurance | Officer to Executive | B.Pharm., M.Pharm. | 5-10 Years | Handle QMS, CAPA, deviations, SOP preparation/execution |
| Production (Liquid & Tablet) | Officer/Sr. Officer | B.Pharm. | 6-8 Years | Manage granulation, compression, coating, liquid manufacturing |
| Production (Liquid & Tablet) | Jr. Officer (Operator) | ITI, Diploma | 7-10 Years | Operate granulation, compression, coating machines, troubleshoot |
| Production (Packing) (Liquid & Tablet) | Officer | B.Pharm. | 5-6 Years | Manage primary/secondary packing, operate BQS, CVC, Elmach |
| Production (Packing) (Liquid & Tablet) | Jr. Officer (Operator) | ITI, Diploma | 7-10 Years | Operate packing machines, ensure bilayer product operations |
Warehouse
- Officer:
- Manage raw material receiving, dispensing, and packing material activities.
- Ensure accurate documentation and compliance with GMP standards.
- Skills: Strong communication, proficiency in warehouse operations.
Quality Control
- Officer to Executive:
Quality Assurance
- Officer to Executive:
Production (Liquid & Tablet)
- Officer/Sr. Officer:
- Manage granulation, compression, coating, capsule, and liquid manufacturing operations.
- Ensure QMS compliance and accurate documentation.
- Skills: Expertise in machine operations, bilayer product handling.
- Jr. Officer (Operator):
Production (Packing) (Liquid & Tablet)
- Officer:
- Manage primary and secondary packing for tablets, capsules, and liquids.
- Oversee operations of BQS, Elmach 3015/3000, CVC labeling, and Monoblock SPR.
- Skills: Knowledge of track-and-trace, serialization, and GMP compliance.
- Jr. Officer (Operator):
Candidate Requirements
- Qualifications: B.Pharm., M.Pharm., B.Sc., M.Sc. (Chemistry), ITI, Diploma (Mechanical/Electrical).
- Experience: 4-10 years (specific to role; see table).
- Preferences:
- Experience in USFDA/MHRA-regulated plants.
- Proficiency in cGMP/GLP documentation and QMS processes.
- Strong communication and technical skills.
- Work Location: Pithampur, Madhya Pradesh.
Application Requirements
- Updated resume
- Current CTC and expected CTC
- Notice period details
- Educational certificates (optional for initial application)
Why Join Alembic Pharmaceuticals?
Established in 1907, Alembic Pharmaceuticals is a trusted name in global healthcare, with a 3.8/5 rating on AmbitionBox for job security. Our Pithampur facility, part of the Smart Industrial Park, offers cutting-edge infrastructure and opportunities for growth. With a legacy of innovation and a presence in 70+ countries, Alembic fosters a culture of excellence and integrity. Learn more at Alembic Pharmaceuticals’ website.
How to Apply
Email your updated resume, current CTC, expected CTC, and notice period to vivek.amishra@alembic.co.in or pratiksha.dubey@alembic.co.in, with the subject line “CV for [Position]”. Ensure you specify the role (e.g., “CV for Warehouse Officer”). Candidates unable to apply immediately can connect via LinkedIn for updates. Caution: Alembic does not charge fees or use consultants for hiring; beware of fraudulent offers.