Alentris Research Private Limited, a specialized Contract Research Organization (CRO) focusing on API impurities and analytical standards, is hosting a walk-in interview for its facility in Gandhinagar, Gujarat. Join our innovative team to advance pharmaceutical research and quality assurance.
Contents
About Alentris Research Pvt. Ltd.
Founded in 2017, Alentris Research Pvt. Ltd. excels in providing solutions for pharmaceutical and biopharmaceutical companies, specializing in API impurities, degradation studies, and regulatory compliance (EP, USP). Located in Gandhinagar, our facility supports cutting-edge research. Learn more at Alentris Research.
Open Positions
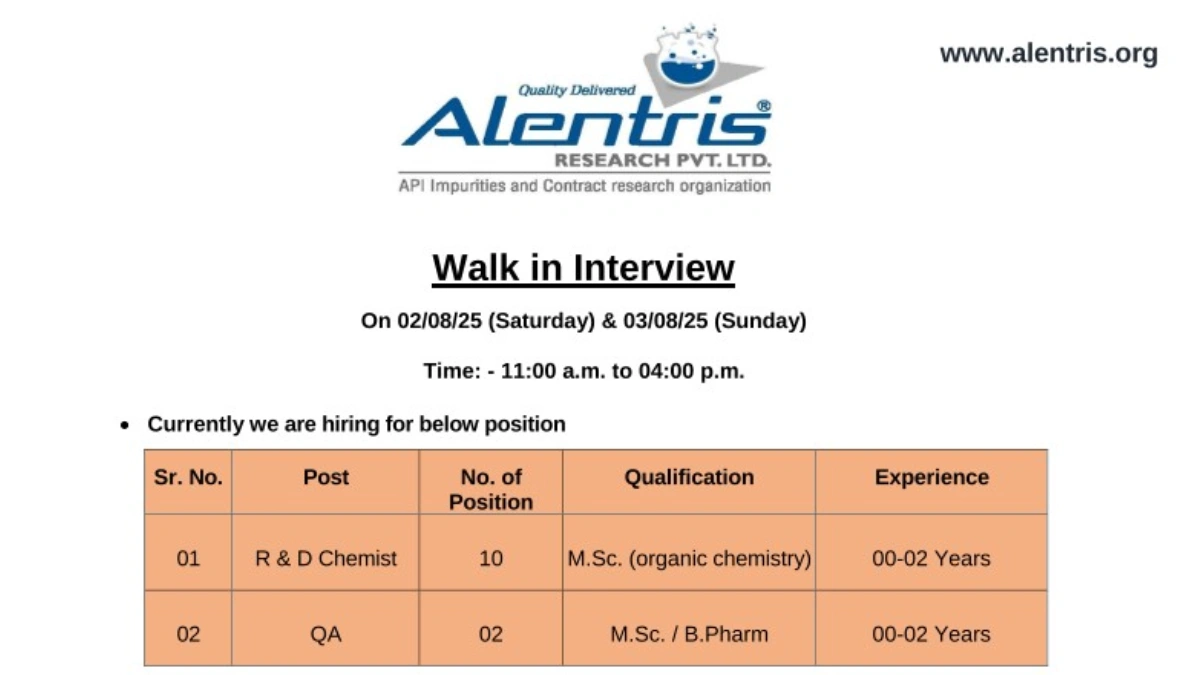
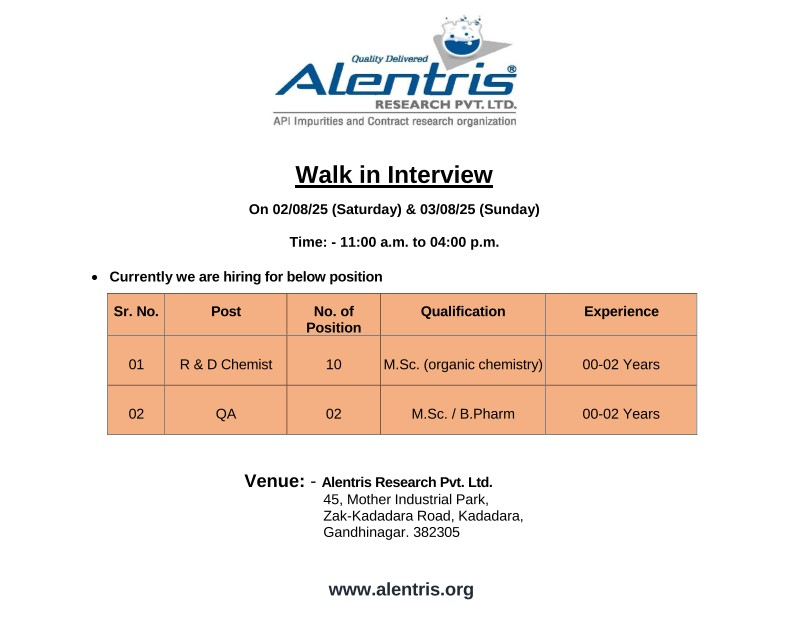
We are hiring for the following roles, open to freshers and candidates with up to 2 years of experience:
R&D Chemist
- Post: R&D Chemist
- No. of Positions: 10
- Qualification: M.Sc. (Organic Chemistry)
- Experience: 0-2 years
- Location: Kadadara, Gandhinagar, Gujarat
Skills:
- Knowledge of organic synthesis, API impurity profiling, and analytical techniques (e.g., HPLC, GC, NMR).
- Familiarity with forced degradation studies (photolytic, thermal) and method development.
- Ability to document experiments and support regulatory compliance (EP, USP).
Quality Assurance (QA)
- Post: QA
- No. of Positions: 2
- Qualification: M.Sc. / B.Pharm
- Experience: 0-2 years
- Location: Kadadara, Gandhinagar, Gujarat
Skills:
- Understanding of Quality Management Systems (QMS) and cGMP guidelines.
- Experience in reviewing analytical data, Certificates of Analysis (CoA), and documentation.
- Knowledge of regulatory requirements for API manufacturing.
Walk-In Interview Details
| Date | 2nd & 3rd August, 2025 (Saturday & Sunday) |
| Time | 11:00 AM to 4:00 PM |
| Venue | Alentris Research Pvt. Ltd., Plot No. 45, Mother Industrial Park, Zak-Kadadara Road, Kadadara, Gandhinagar, Gujarat – 382305 |
| Contact | Email: hr@alentris.org |
Candidates unable to attend can email their CV to hr@alentris.org.
Why Join Alentris Research?
Alentris offers a dynamic R&D environment with a focus on impurity science and regulatory compliance. Our Gandhinagar facility provides access to advanced analytical tools, fostering skill development for freshers and early-career professionals.
Eligibility Criteria
- M.Sc. (Organic Chemistry) for R&D Chemist; M.Sc. or B.Pharm for QA.
- 0-2 years of experience; freshers with relevant academic projects welcome.
- Basic knowledge of HPLC, GC, QMS, or cGMP practices.
- Strong analytical and documentation skills.
How to Prepare for the Interview
To succeed, candidates should:
- Bring an updated CV, educational certificates, and mark sheets.
- Be prepared to discuss organic chemistry concepts (R&D) or QMS/cGMP practices (QA).
- Highlight any exposure to API impurity studies or analytical techniques.
- Arrive punctually at the Gandhinagar venue.
Verified by Trusted HRs
The post is released by the Alentris Research Pvt Ltd LinkedIn page. Click here to visit the post
Why Kadadara, Gandhinagar?
Kadadara’s Mother Industrial Park is a growing hub for pharmaceutical research, hosting Alentris’ advanced CRO facility, ideal for professionals in API and analytical development.
Application Process
Attend the walk-in interview on 2nd-3rd August 2025 with required documents or email CVs to hr@alentris.org. Visit Alentris Research for more details.
Career Growth at Alentris
Alentris supports career growth through hands-on training in API impurity profiling and QA processes, offering opportunities to work on projects compliant with global standards.
Note
Alentris does not charge application fees; beware of fraudulent schemes. Freshers and candidates with up to 2 years of experience are encouraged to apply.
Contact Us
For queries, email hr@alentris.org or call +91 9924634390. Join Alentris and drive innovation in pharmaceutical research!