Amgen, a pioneer in biotechnology since 1980, harnesses biology and technology to combat the world’s toughest diseases, improving millions of lives globally. We are hiring an Associate Regulatory Affairs professional for our Hyderabad facility to support FDA 2253 reporting and digital asset management. Join our innovative team to drive regulatory excellence and advance healthcare solutions!
Contents
Job Details
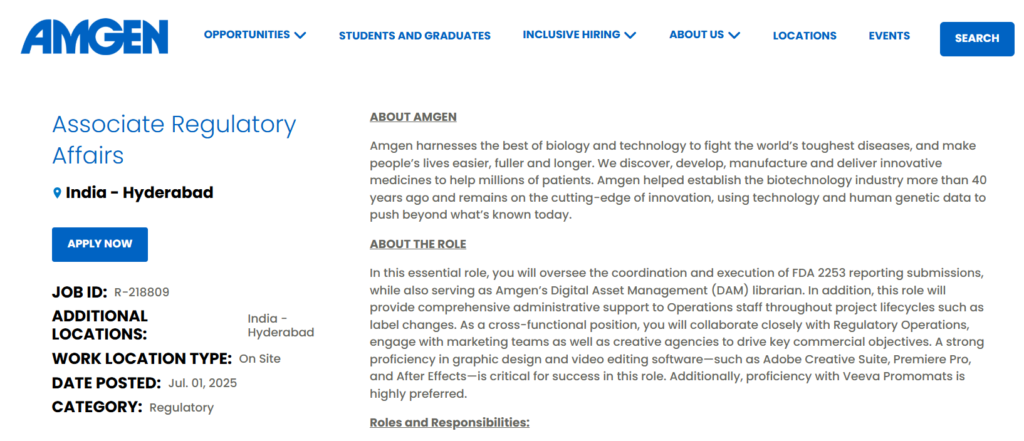
- Job ID: R-218809
- Category: Regulatory
- Location: Hyderabad, Telangana, India
- Work Type: On-Site
- Date Posted: July 1, 2025
- Apply: Amgen Careers
- Work Hours: Flexible AIN hours, with occasional overnight work to support US product launches and new indications
Job Role: Associate Regulatory Affairs
This cross-functional role involves FDA 2253 submissions, Digital Asset Management (DAM), and administrative support for regulatory operations. Below are the details:
Qualifications
- Education: Bachelor’s degree in graphic design, visual arts, marketing, or related field
- Experience: Minimum 2+ years in marketing, pharmaceutical, or healthcare industries
- Certifications: Veeva Business Admin certification preferred
Required Skills:
- Proficiency in Veeva PromoMats, RIM, and digital asset management
- Strong knowledge of Adobe Creative Suite (Photoshop, Illustrator, InDesign), Premiere Pro, and After Effects
- Expertise in Microsoft Office Suite (PowerPoint, Visio, Excel with pivot tables)
- Familiarity with US FDA eCTD publishing tools and FDA 2253 submission guidelines
- Experience with SharePoint, Webex, MS Teams, and metadata management
Preferred Skills:
- Understanding of FDA regulations for promotional materials
- Experience with creative agencies and cross-functional teams
- Strong analytical and problem-solving abilities
Key Responsibilities
- Conduct quality assurance for FDA Form 2253 submissions for promotional materials.
- Evaluate creative file submissions and metadata for accuracy and compliance.
- Collaborate with marketing teams and creative agencies to manage usage rights.
- Provide art procurement services using Amgen’s subscriptions.
- Retrieve, validate, and distribute digital assets via Veeva, SharePoint, and BOX.
- Maintain records in Veeva PromoMats, RIM, and SharePoint.
- Lead stakeholder meetings, prepare agendas, and document minutes.
- Create infographics, presentations, and process documentation.
- Design and run reports using Veeva’s reporting tools for performance analysis.
- Manage MS Outlook inbox with organizational tools like email rules and templates.
Why Join Amgen?
Amgen is a global biotechnology leader with a 4.1/5 employee rating on Glassdoor for innovation and work culture. Our Hyderabad facility supports cutting-edge regulatory operations. Here’s why you should join us:
- Innovative Environment: Work with advanced biotech and digital tools.
- Career Growth: Access robust training and development opportunities.
- Global Impact: Support medicines that transform lives worldwide.
- Inclusive Culture: Thrive in an equitable, diverse workplace with reasonable accommodations.
How to Apply
To apply, submit the following via Amgen Careers by July 31, 2025:
- Updated resume highlighting Veeva PromoMats and regulatory experience.
- Cover letter explaining your fit for the role.
- Proof of Veeva Business Admin certification (if applicable).
- Copies of educational certificates and ID proof (Aadhar, PAN).
- Portfolio showcasing Adobe Creative Suite work (optional).
Verified by Trusted HRs
The post is released by the Amgen Official Webpage. Click here to visit the post
About Amgen
Amgen, headquartered in Thousand Oaks, California, is a global biotechnology pioneer with a presence in 100+ countries. Our Hyderabad center drives regulatory and operational excellence, supporting innovative therapies in oncology, cardiology, and more. Learn more at www.amgen.com.
Equal Opportunity Statement
Amgen is an Equal Opportunity employer, considering candidates regardless of race, color, religion, sex, sexual orientation, gender identity, national origin, veteran status, or disability. We provide reasonable accommodations for individuals with disabilities. Contact us at careers@amgen.com to request support.
Join Amgen to shape the future of biotechnology. Apply now and make a difference in global healthcare from Hyderabad!