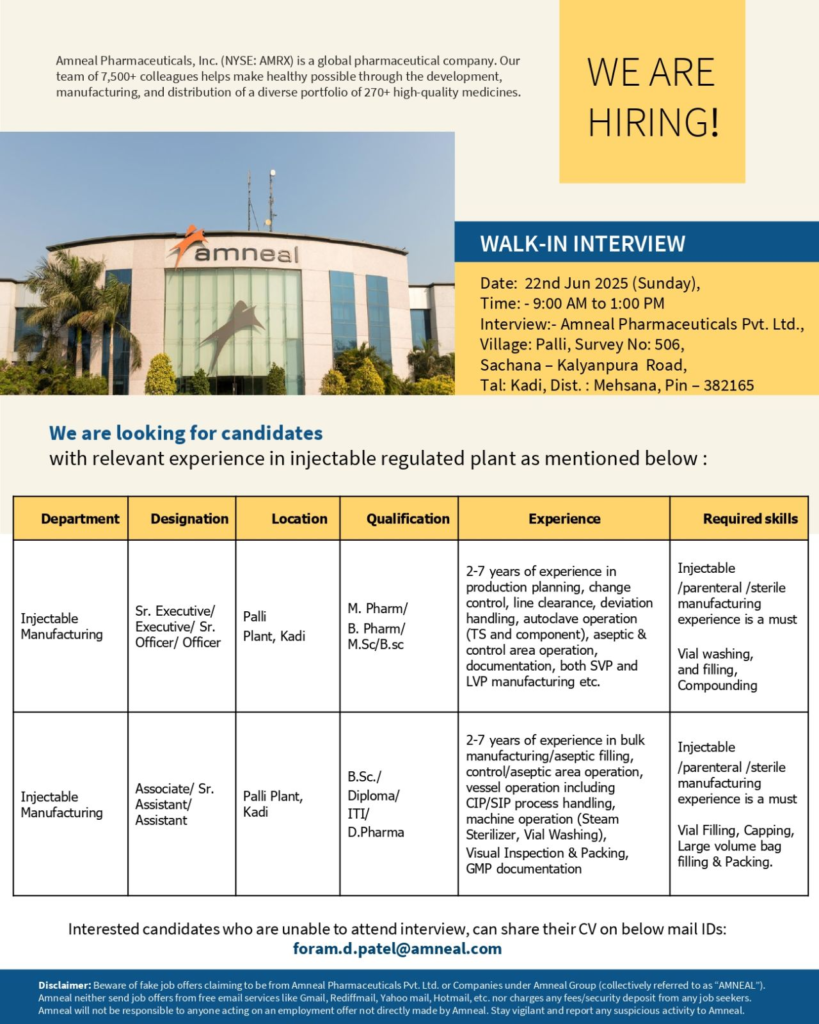
Join Amneal Pharmaceuticals Pvt. Ltd., a global leader with 7,500+ colleagues and a portfolio of 270+ high-quality medicines, at our walk-in interview on June 22, 2025, for roles in Injectable Manufacturing at our USFDA-approved Palli Plant in Kadi, Mehsana, Gujarat. We are seeking experienced professionals to support our sterile and parenteral production operations.
Event Details
- Date: Sunday, June 22, 2025
- Time: 9:00 AM to 1:00 PM IST
- Venue: Amneal Pharmaceuticals Pvt. Ltd., Village: Palli, Survey No: 506, Sachana-Kalyanpura Road, Tal: Kadi, Dist.: Mehsana, Gujarat 382165
- Note: Bring updated CV, educational certificates, last 3 months’ payslips, Aadhaar/PAN ID proof, and one passport-size photo. Candidates must have experience in injectable/parenteral manufacturing in a regulated plant.
Job Opportunities
| Department | Designation | Location | Qualification | Experience | Required Skills |
|---|---|---|---|---|---|
| Injectable Manufacturing | Sr. Executive / Executive / Sr. Officer / Officer | Palli Plant, Kadi | M.Pharm / B.Pharm / M.Sc / B.Sc | 2-7 Yrs | Production planning, change control, line clearance, deviation handling, autoclave operation (TS and component), aseptic & controlled area operations, documentation, SVP and LVP manufacturing, vial washing, filling, compounding |
| Injectable Manufacturing | Associate / Sr. Assistant / Assistant | Palli Plant, Kadi | B.Sc / Diploma / ITI / D.Pharm | 2-7 Yrs | Bulk manufacturing, aseptic filling, controlled/aseptic area operations, vessel operation (CIP/SIP), machine operation (steam sterilizer, vial washing), visual inspection, packing, GMP documentation, vial filling, capping, large volume bag filling & packing |
Key Responsibilities
Sr. Executive / Executive / Sr. Officer / Officer (Injectable Manufacturing):
- Oversee production planning, change control, and deviation handling for small volume parenterals (SVP) and large volume parenterals (LVP)
- Manage autoclave operations (terminal sterilization and component), vial washing, filling, and compounding
- Ensure aseptic and controlled area operations comply with cGMP and USFDA standards
- Maintain accurate documentation (BMR/BPR) and support regulatory audits
Associate / Sr. Assistant / Assistant (Injectable Manufacturing):
- Perform bulk manufacturing, aseptic filling, and vial washing/filling/capping
- Operate vessels (CIP/SIP) and machines (steam sterilizer, vial washing)
- Conduct visual inspections, large volume bag filling, and packing
- Ensure GMP-compliant documentation and adherence to aseptic protocols
Desired Profile
- 2-7 years of experience in injectable/parenteral/sterile manufacturing in USFDA-regulated plants
- Strong knowledge of cGMP, GDP, ALCOA, and aseptic operations
- Hands-on experience with vial washing, filling, capping, autoclave, and compounding
- Familiarity with QMS, change control, deviation handling, and GMP documentation
- Ability to work in shifts and maintain audit readiness
- Good communication and teamwork skills
Why Join Amneal Pharmaceuticals?
Amneal Pharmaceuticals, founded in 2002, is a global leader in generics and injectables, rated 4.1/5 on AmbitionBox for work culture and 4.2/5 for work-life balance. Our Palli Plant in Kadi, Mehsana, is USFDA-approved and focuses on sterile manufacturing, offering robust training and career growth (rated 4.0/5 for skill development). Employees benefit from a dynamic environment, though some note variable increments (3.8/5 for salary). Learn more at Amneal Pharmaceuticals.
How to Apply
- Walk-In: Attend with required documents on June 22, 2025.
- Email: Send CV to foram.d.patel@amneal.com.
- Include: Updated CV, educational certificates, and details of current CTC, expected CTC, and notice period.
Note: USFDA-regulated plant experience mandatory. No fees charged.
Additional Information
Explore pharmaceutical career trends at PharmaVoice. Join Amneal to make healthy possible through innovative manufacturing!
Disclaimer: Amneal Pharmaceuticals does not use free email services (e.g., Gmail, Yahoo) for job offers or charge fees. Verify offers through official channels (e.g., careers@amneal.com) and report suspicious activity.
Note: Shortlisting based on qualifications, injectable manufacturing experience, and USFDA exposure.
