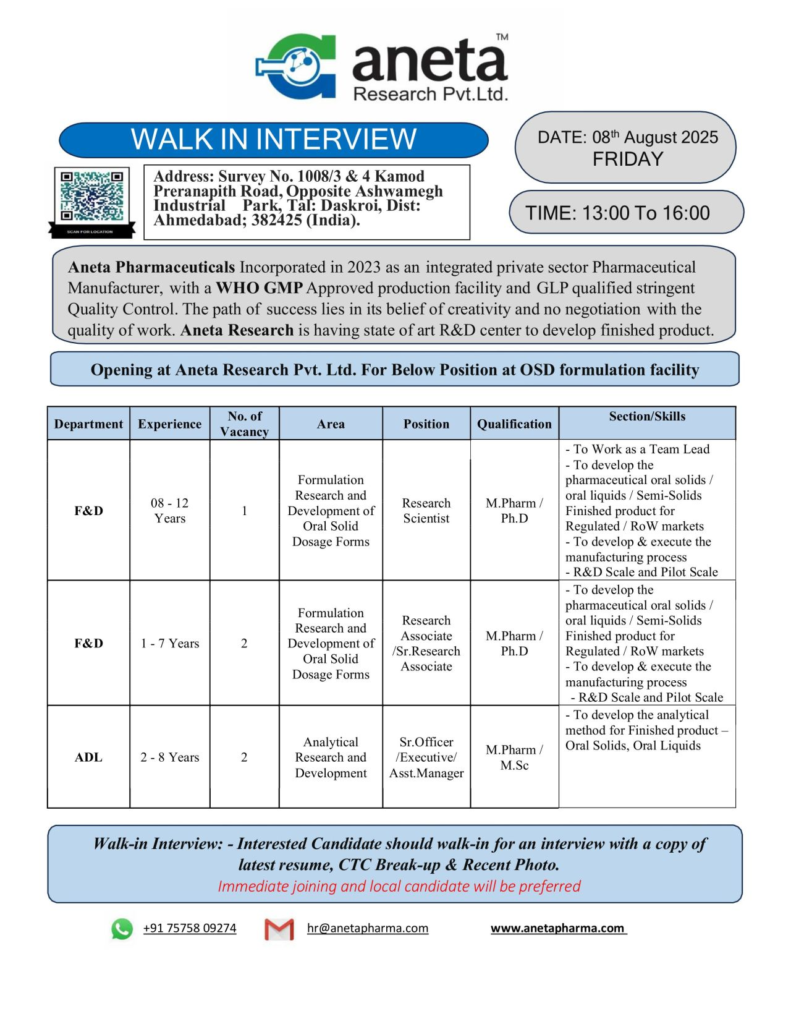
Aneta Research Pvt. Ltd., a rapidly growing pharmaceutical company established in 2023, is hosting a walk-in interview on August 8, 2025, for talented professionals to join our state-of-the-art Oral Solid Dosage (OSD) Formulation Facility in Ahmedabad.
With a WHO-GMP approved production facility and a GLP-qualified quality control system, we are committed to innovation, quality, and excellence in pharmaceutical research and development. Join us to contribute to cutting-edge healthcare solutions for regulated and ROW markets.
Contents
- 1 About Aneta Research Pvt. Ltd.
- 2 Open Positions in Formulation and Analytical Development
- 3 Why Join Aneta Research Pvt. Ltd.?
- 4 Walk-In Interview Details
- 5 How to Apply
- 6 Why Ahmedabad?
- 7 Join Our Mission
About Aneta Research Pvt. Ltd.
Aneta Research Pvt. Ltd. is an integrated pharmaceutical manufacturer based in Ahmedabad, Gujarat, specializing in the development and production of high-quality generic medicines, including tablets, capsules, injectables, and ointments.
Our advanced R&D center is equipped to develop innovative formulations, ensuring compliance with global regulatory standards. With a workforce of over 188 professionals and a focus on creativity and quality, we are poised for significant growth in the pharmaceutical industry.
Open Positions in Formulation and Analytical Development
We are seeking passionate individuals for the following roles in our Formulation Research and Development (F&D) and Analytical Development (ADL) departments at our OSD formulation facility.
1. Research Scientist – Formulation Research and Development (F&D)
Role Overview
As a Research Scientist, you will lead a team in developing pharmaceutical oral solids, liquids, and semi-solids for regulated and Rest of World (ROW) markets. This role requires expertise in formulation development and process execution at R&D and pilot scales.
Key Responsibilities
- Lead a team in formulation development for oral solids, liquids, and semi-solids.
- Develop and execute manufacturing processes for R&D and pilot-scale production.
- Ensure compliance with regulatory standards for global markets.
- Collaborate with cross-functional teams to optimize product development.
Qualifications and Skills
| Criteria | Details |
|---|---|
| Qualification | M.Pharm or Ph.D |
| Experience | 8-12 years in OSD formulation development |
| No. of Vacancies | 1 |
| Key Skills | Team leadership, process development, regulatory compliance, OSD expertise |
| Section | Formulation Research and Development (Oral Solid Dosage Forms) |
2. Research Associate / Senior Research Associate – Formulation Research and Development (F&D)
Role Overview
The Research Associate/Sr. Research Associate role involves developing pharmaceutical formulations and supporting manufacturing processes for oral solids, liquids, and semi-solids. This position is ideal for professionals eager to contribute to innovative drug development.
Key Responsibilities
- Develop formulations for oral solids, liquids, and semi-solids for regulated/ROW markets.
- Execute manufacturing processes at R&D and pilot scales.
- Support process optimization and scale-up activities.
- Collaborate with analytical teams to ensure product quality.
Qualifications and Skills
| Criteria | Details |
|---|---|
| Qualification | M.Pharm or Ph.D |
| Experience | 1-7 years in OSD formulation development |
| No. of Vacancies | 2 |
| Key Skills | Formulation development, process execution, OSD expertise |
| Section | Formulation Research and Development (Oral Solid Dosage Forms) |
3. Sr. Officer / Executive / Assistant Manager – Analytical Research and Development (ADL)
Role Overview
The Analytical Development role focuses on developing and validating analytical methods for finished products, including oral solids and liquids. This position ensures product quality through rigorous testing and compliance with regulatory standards.
Key Responsibilities
- Develop and validate analytical methods for oral solids and liquids.
- Conduct testing to ensure product quality and compliance.
- Support formulation teams with analytical insights.
- Maintain GLP-compliant documentation and processes.
Qualifications and Skills
| Criteria | Details |
|---|---|
| Qualification | M.Pharm or M.Sc |
| Experience | 2-8 years in analytical development |
| No. of Vacancies | 2 |
| Key Skills | Analytical method development, GLP compliance, quality testing |
| Section | Analytical Research and Development |
Why Join Aneta Research Pvt. Ltd.?
Aneta Research Pvt. Ltd. is dedicated to delivering high-quality pharmaceutical solutions with a focus on innovation and patient care. Here’s why you should join us:
- State-of-the-Art Facility: Work in a WHO-GMP approved R&D center with advanced equipment for formulation and analytical development.
- Career Growth: Gain hands-on experience in developing products for regulated markets under expert mentorship.
- Innovative Culture: Contribute to creative solutions in a collaborative and quality-driven environment.
- Local Preference: Immediate joiners and candidates from Ahmedabad are preferred for seamless onboarding.
Walk-In Interview Details
- Date: Friday, August 8, 2025
- Time: 1:00 PM to 4:00 PM
- Venue: Aneta Research Pvt. Ltd., Survey No. 1008/3 & 4, Kamod Preranapith Road, Opposite Ashwamegh Park, Daskroi, Ahmedabad, Gujarat – 382425, India
What to Bring:
- Updated resume
- CTC break-up (if applicable)
- Recent passport-size photograph
- Educational certificates and ID proof
How to Apply
Attend the walk-in interview with the required documents to explore these exciting opportunities. Unable to attend? Send your resume to hr@anetapharma.com or contact us at +91 75758 09274. For more details about our work, visit Aneta Research Pvt. Ltd.’s official website.
Why Ahmedabad?
Ahmedabad is a thriving hub for pharmaceutical innovation, offering a dynamic environment for career growth. Our facility in Daskroi is strategically located, providing access to cutting-edge R&D and manufacturing processes in a vibrant industrial ecosystem.
Join Our Mission
At Aneta Research Pvt. Ltd., we believe in making life healthy through innovative pharmaceutical solutions. Attend our walk-in interview on August 8, 2025, and become part of a team dedicated to excellence in formulation research and development. We look forward to meeting you!


