Join Anthea Pharma, a leading injectable pharmaceutical manufacturer, at our USFDA-compliant facility in Pashamylaram, Telangana. We’re hiring for Quality Assurance (IPQA) roles to ensure cGMP compliance and drive quality excellence in sterile manufacturing. Be part of a dynamic team dedicated to delivering high-quality injectables globally.
About Anthea Pharma
Anthea Pharma specializes in sterile injectables, including lyophilized products, with a focus on regulatory compliance and innovation. Our Pashamylaram facility adheres to USFDA, MHRA, and cGMP guidelines, making us a trusted partner in the global pharmaceutical industry. We emphasize quality assurance to ensure patient safety and product efficacy.
Job Opportunities in Quality Assurance (IPQA)
We’re seeking candidates with injectable manufacturing experience for QA-IPQA roles at our Pashamylaram facility. Candidates must have strong documentation, communication, and regulatory knowledge to support aseptic processes and quality systems.
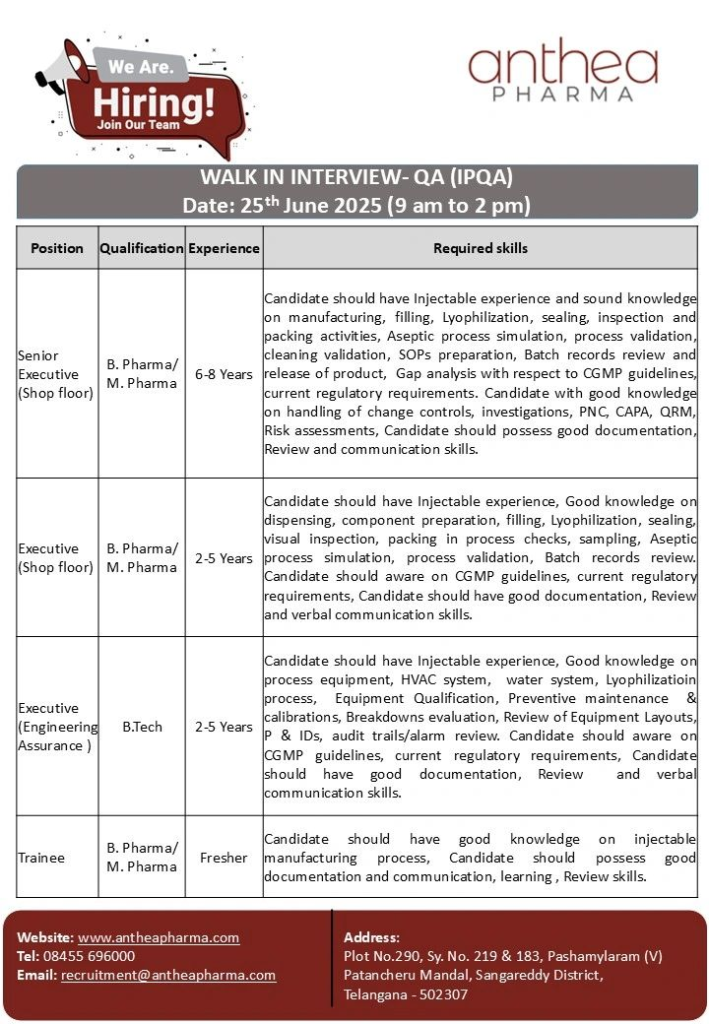
Senior Executive (Shop Floor)
- Qualification: B.Pharmacy/M.Pharmacy
- Experience: 6-8 Years
- Required Skills:
- Expertise in injectable manufacturing, filling, lyophilization, sealing, inspection, and packing.
- Proficient in aseptic process simulation, process validation, and cleaning validation.
- Skilled in SOP preparation, batch record review, and product release.
- Knowledge of cGMP guidelines, change controls, CAPA, QRM, and risk assessments.
- Strong documentation, review, and communication skills.
- Responsibilities: Conduct gap analysis for cGMP compliance, handle investigations, PNC, and ensure regulatory readiness.
Executive (Shop Floor)
- Qualification: B.Pharmacy/M.Pharmacy
- Experience: 2-5 Years
- Required Skills:
- Experience in dispensing, component preparation, filling, lyophilization, sealing, visual inspection, and packing.
- Knowledge of in-process checks, sampling, aseptic process simulation, and process validation.
- Familiarity with cGMP guidelines and batch record review.
- Good documentation, review, and verbal communication skills.
- Responsibilities: Perform IPQA checks, ensure cGMP adherence, and support batch documentation.
Executive (Engineering Assurance)
- Qualification: B.Tech
- Experience: 2-5 Years
- Required Skills:
- Knowledge of process equipment, HVAC systems, water systems, and lyophilization processes.
- Expertise in equipment qualification, preventive maintenance, calibrations, and breakdown evaluations.
- Skilled in reviewing equipment layouts, P&IDs, audit trails, and alarms.
- Awareness of cGMP guidelines and regulatory requirements.
- Strong documentation, review, and verbal communication skills.
- Responsibilities: Oversee engineering QA, ensure equipment compliance, and support validation activities.
Trainee
- Qualification: B.Pharmacy/M.Pharmacy
- Experience: Fresher
- Required Skills:
- Basic understanding of injectable manufacturing processes.
- Strong documentation, communication, learning, and review skills.
- Responsibilities: Assist in IPQA activities, learn cGMP standards, and support quality documentation.
Interview Details
- Date: Wednesday, June 25, 2025
- Time: 9:00 AM – 2:00 PM
- Venue: Anthea Pharma, Plot No. 290, Sy. No. 219 & 183, Pashamylaram (V), Patancheru Mandal, Sangareddy District, Telangana – 502307
- Job Location: Pashamylaram, Telangana
- Requirements: Bring updated resume, educational certificates, Aadhaar/PAN card, and recent payslips (if applicable)
- Contact: Call +91 8455 696000 or email CV to recruitment@antheapharma.com
- Website: www.antheapharma.com
Why Join Anthea Pharma?
- Global Impact: Contribute to high-quality injectable production for international markets.
- Advanced Facility: Work in a USFDA/MHRA-compliant facility with cutting-edge lyophilization and aseptic technologies.
- Career Growth: Gain exposure to regulatory audits, QMS, and validation processes in Telangana’s pharma hub.
- Supportive Culture: Join a team that values innovation, collaboration, and continuous learning.
- Fresher Opportunities: Kickstart your career with hands-on training in injectable QA.
Important Notes
- Candidates must have injectable experience (except for trainees) in USFDA/MHRA-regulated plants.
- cGMP knowledge and documentation skills are mandatory for all roles.
- Freshers must demonstrate learning aptitude and basic injectable process knowledge.
- Candidates interviewed at Anthea in the last 6 months should not apply.
- Disclaimer: Anthea Pharma does not charge fees for recruitment. Report suspicious activities.
How to Prepare for the Interview
- Experienced Candidates: Highlight your injectable experience, cGMP expertise, and specific achievements in IPQA, validation, or audit compliance on your CV. Be ready to discuss change controls, CAPA, or risk assessments.
- Freshers: Emphasize your academic knowledge of injectable processes and communication skills. Prepare to showcase your willingness to learn and attention to detail.
- Bring all required documents to ensure a smooth interview process.
Apply now to advance your career in injectable quality assurance with Anthea Pharma. Visit www.antheapharma.com to learn more about our mission to deliver world-class pharmaceuticals.

