Seize pharma jobs at APDM! Attend walk-in interviews for QC jobs, QA roles, production jobs, and engineering in pharmaceutical careers in India. Regulatory exposure required—apply now in Ahmedabad.
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Quality Control Officer / Sr. Officer
- 3.2 Quality Control Executive / Sr. Executive
- 3.3 Quality Control Sr. Executive / Assistant Manager
- 3.4 Quality Control Executive / Sr. Executive (AQA)
- 3.5 Quality Assurance Executive / Sr. Executive / Assistant Manager (IPQA)
- 3.6 Quality Assurance Executive / Sr. Executive (Documentation)
- 3.7 Quality Assurance Executive / Sr. Executive (Reviewer Qualification)
- 3.8 Quality Assurance Sr. Executive / Assistant Manager (Reviewer QMS)
- 3.9 Quality Assurance Executive / Sr. Executive (Reviewer Validation)
- 3.10 Instrumentation Technician
- 3.11 Engineering Officer
- 3.12 Fitter (Process Equipment)
- 3.13 Operator (HVAC/Utility)
- 3.14 Electrician
- 3.15 Operator (RO)
- 3.16 Production Executive / Sr. Executive (Packaging)
- 3.17 Production Officer / Sr. Officer (Tablet & Capsule)
- 3.18 Production Assistant Manager
- 3.19 Production Operator (Granulation, Coating & Capsule, Compression, Packaging)
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
APDM Pharmaceuticals Pvt. Ltd., founded in June 2022 in Ahmedabad, Gujarat, is a dynamic contract development and manufacturing organization (CDMO) specializing in solid oral formulations.
Driven by visionary entrepreneurs and seasoned scientists, the company emphasizes innovation, regulatory compliance with EU, MHRA, USFDA, and ANVISA standards, and sustainable growth in the global pharma sector.
As a closely held entity, APDM fosters credibility through advanced R&D and client-focused solutions, exporting quality generics while building a robust presence in regulated markets.
Job Details
- Company Name: APDM Pharmaceuticals
- Experience: 2 to 12 Years (varies by role)
- Qualification: M.Sc./M.Pharm/B.Pharm, ITI/Diploma/BE, 12th Pass
- Location: Sakodara Village, Ahmedabad, Gujarat
- Work Type: On-site
Job Description
APDM Pharmaceuticals is conducting a multi-day walk-in drive to recruit for its expanding operations in quality, production, and engineering. These pharma jobs demand regulatory exposure to EU, MHRA, USFDA, and ANVISA audits. Join a innovative CDMO advancing pharmaceutical careers in India through solid oral expertise.
Quality Control Officer / Sr. Officer
- Department: Quality Control
- Role: Packing Material & Chemical Analysis
- Experience: 2 to 5 Years
- Education/Qualification: M.Sc./M.Pharm
Quality Control Executive / Sr. Executive
- Department: Quality Control
- Role: RM Sampling & Instrumental Analysis
- Experience: 4 to 6 Years
- Education/Qualification: M.Sc./M.Pharm
Quality Control Sr. Executive / Assistant Manager
- Department: Quality Control
- Role: AMT Planning & GLP Compliance
- Experience: 6 to 10 Years
- Education/Qualification: M.Sc./M.Pharm
Quality Control Executive / Sr. Executive (AQA)
- Department: Quality Control
- Role: In-Process & QMS Activities
- Experience: 7 to 9 Years
- Education/Qualification: M.Sc./M.Pharm
Quality Assurance Executive / Sr. Executive / Assistant Manager (IPQA)
- Department: Quality Assurance
- Role: BMR Review & Batch Release
- Experience: 6 to 9 Years
- Education/Qualification: M.Sc./B.Pharm/M.Pharm
Quality Assurance Executive / Sr. Executive (Documentation)
- Department: Quality Assurance
- Role: BMR Issuance & Doc Management
- Experience: 6 to 8 Years
- Education/Qualification: M.Sc./B.Pharm/M.Pharm
Quality Assurance Executive / Sr. Executive (Reviewer Qualification)
- Department: Quality Assurance
- Role: Calibration & HVAC Validation
- Experience: 5 to 7 Years
- Education/Qualification: M.Sc./B.Pharm/M.Pharm
Quality Assurance Sr. Executive / Assistant Manager (Reviewer QMS)
- Department: Quality Assurance
- Role: QMS Document Review
- Experience: 7 to 9 Years
- Education/Qualification: M.Sc./B.Pharm/M.Pharm
Quality Assurance Executive / Sr. Executive (Reviewer Validation)
- Department: Quality Assurance
- Role: Product Launch Documentation
- Experience: 6 to 8 Years
- Education/Qualification: M.Sc./B.Pharm/M.Pharm
Instrumentation Technician
- Department: Engineering
- Role: Equipment Breakdown Repairs
- Experience: 3 to 5 Years
- Education/Qualification: ITI/Diploma
Engineering Officer
- Department: Engineering
- Role: HVAC & Water System Maintenance
- Experience: 3 to 5 Years
- Education/Qualification: BE (Any Stream)
Fitter (Process Equipment)
- Department: Engineering
- Role: Utility Equipment Operations
- Experience: 3 to 5 Years
- Education/Qualification: ITI
Operator (HVAC/Utility)
- Department: Engineering
- Role: System Monitoring & Maintenance
- Experience: 4 to 7 Years
- Education/Qualification: ITI
Electrician
- Department: Engineering
- Role: Electrical Systems Troubleshooting
- Experience: 3 to 5 Years
- Education/Qualification: ITI
Operator (RO)
- Department: Engineering
- Role: Water System Operations
- Experience: 3 to 5 Years
- Education/Qualification: ITI
Production Executive / Sr. Executive (Packaging)
- Department: Production
- Role: Packaging Operations Supervision
- Experience: 4 to 7 Years
- Education/Qualification: B.Pharm/M.Pharm
Production Officer / Sr. Officer (Tablet & Capsule)
- Department: Production
- Role: Manufacturing Equipment Operations
- Experience: 2 to 6 Years
- Education/Qualification: B.Pharm/M.Pharm
Production Assistant Manager
- Department: Production
- Role: Tablet & Capsule Supervision
- Experience: 8 to 12 Years
- Education/Qualification: B.Pharm/M.Pharm
Production Operator (Granulation, Coating & Capsule, Compression, Packaging)
- Department: Production
- Role: Machine Operations in Sections
- Experience: 3 to 5 Years
- Education/Qualification: ITI/12th Pass
Skills/Qualifications
- Expertise in HPLC, GC, UV, IR, KF, Polarimeter, Dissolution
- Knowledge of cGMP, GLP, ALCOA++, GDP compliance
- Experience in SOP preparation, MOA, protocols, method transfer
- Regulatory audit exposure (EU, MHRA, USFDA, ANVISA mandatory)
- Proficiency in BMR/BPR review, deviation investigation, OOS/OOT
- Technical skills in HVAC, utilities, electrical, RO systems
- B.Pharm/M.Pharm for QA/QC; ITI/Diploma/BE for engineering/production
Key Responsibilities
- Perform chemical/instrumental analysis daily
- Review and approve QMS documents promptly
- Maintain HVAC/utilities per SOPs
- Execute production batches with cGMP adherence
- Investigate deviations and ensure audit readiness
- Document operations for regulatory compliance
Benefits/Perks
- Strong career progression in CDMO environment
- Continuous training on regulatory standards
- Competitive salary with audit bonuses
- Supportive culture fostering innovation
- Global exposure via international compliance
How to Apply
Attend the walk-in interview with your resume and one passport-size photo. For additional pharma jobs advice, visit Pharma Recruiter. Secure your spot—walk in today for transformative pharmaceutical careers in India!
Verified Post
The post is released by the APDM Pharmaceuticals LinkedIn page. Click here to visit the post
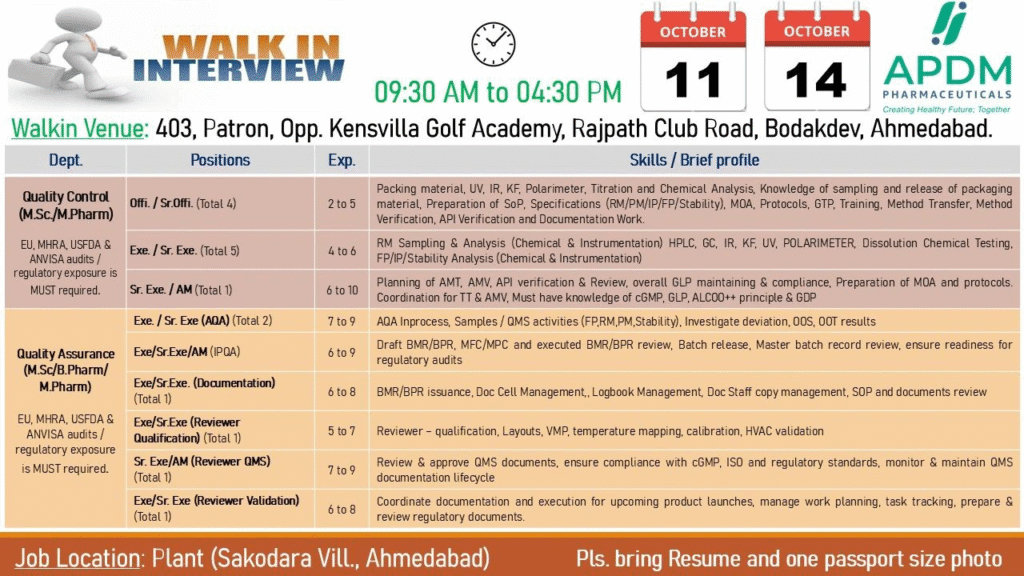


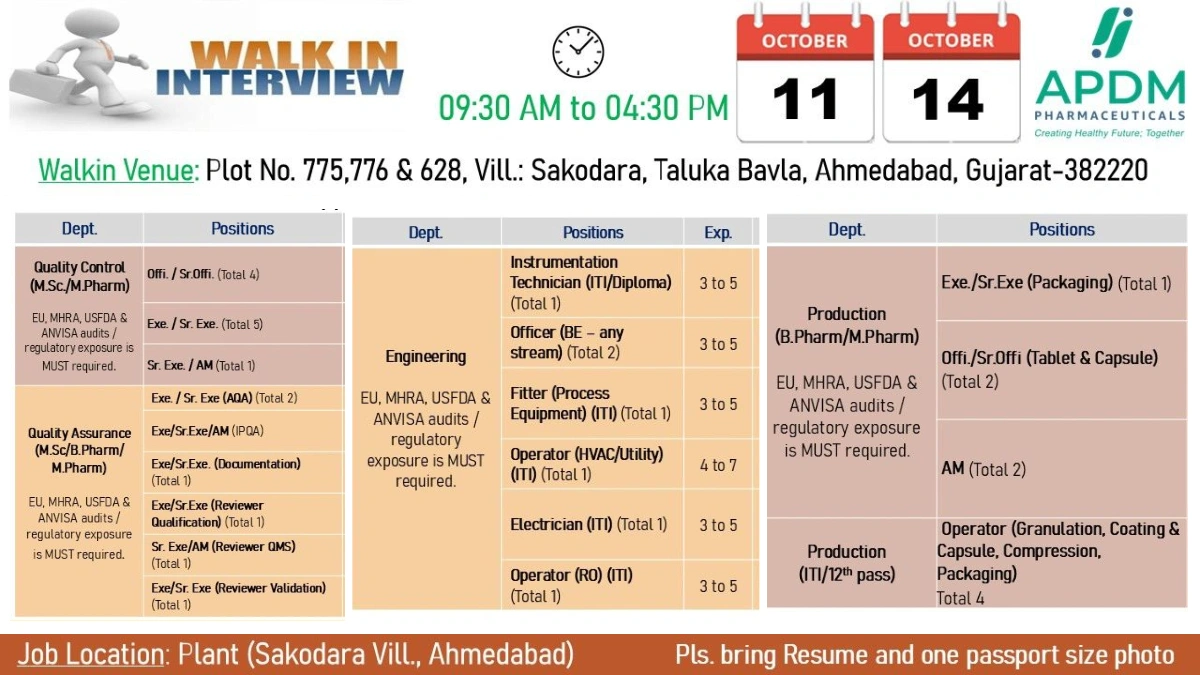
Walk-in Interview Details
- Date: October 11 to 14, 2025
- Time: 09:30 AM to 04:30 PM
- Venue (QC/QA): 403, Patron, Opp. Kensvilla Golf Academy, Rajpath Club Road, Bodakdev, Ahmedabad
- Venue (Engineering/Production): Plot No. 775, 776 & 628, Vill. Sakodara, Taluka Bavla, Ahmedabad, Gujarat-382220
- What to Bring: Resume and one passport-size photo
Why You Should Join
APDM Pharmaceuticals nurtures a culture of collaboration and excellence, recognized for its rapid growth since 2022 in solid orals CDMO. Enjoy long-term stability through regulatory mastery and R&D innovation, with ample learning opportunities in a compliance-centric setting that propels your QA, QC, and production expertise forward.
FAQs
What regulatory experience is required for these pharma jobs?
EU, MHRA, USFDA, and ANVISA audit exposure is mandatory for most QC, QA, and production roles at APDM.
Where are the walk-in interviews held?
QC/QA at Bodakdev venue; Engineering/Production at Sakodara plant—attend based on your department from October 11-14.
Who qualifies for engineering positions?
ITI/Diploma/BE holders with 3-7 years in HVAC, utilities, or electrical maintenance for on-site pharma jobs.
What growth opportunities await at APDM?
From executive to assistant manager roles, with training in cGMP and global CDMO projects enhancing pharmaceutical careers in India.