Aurobindo Pharma Ltd., a global pharmaceutical leader founded in 1986, is hosting a walk-in interview for its Unit VII (OSD) facility in Jadcherla, Telangana. With 26 manufacturing plants and a presence in 150+ countries, Aurobindo is renowned for its USFDA, EU-GMP, and WHO-GMP compliant operations, producing generics and APIs.
Rated 3.9/5 on AmbitionBox for job security, we invite professionals with 2–6 years of experience to join our team of 15,000+ employees to drive excellence in oral solid dosage (OSD) manufacturing.
Contents
Walk-In Interview Details
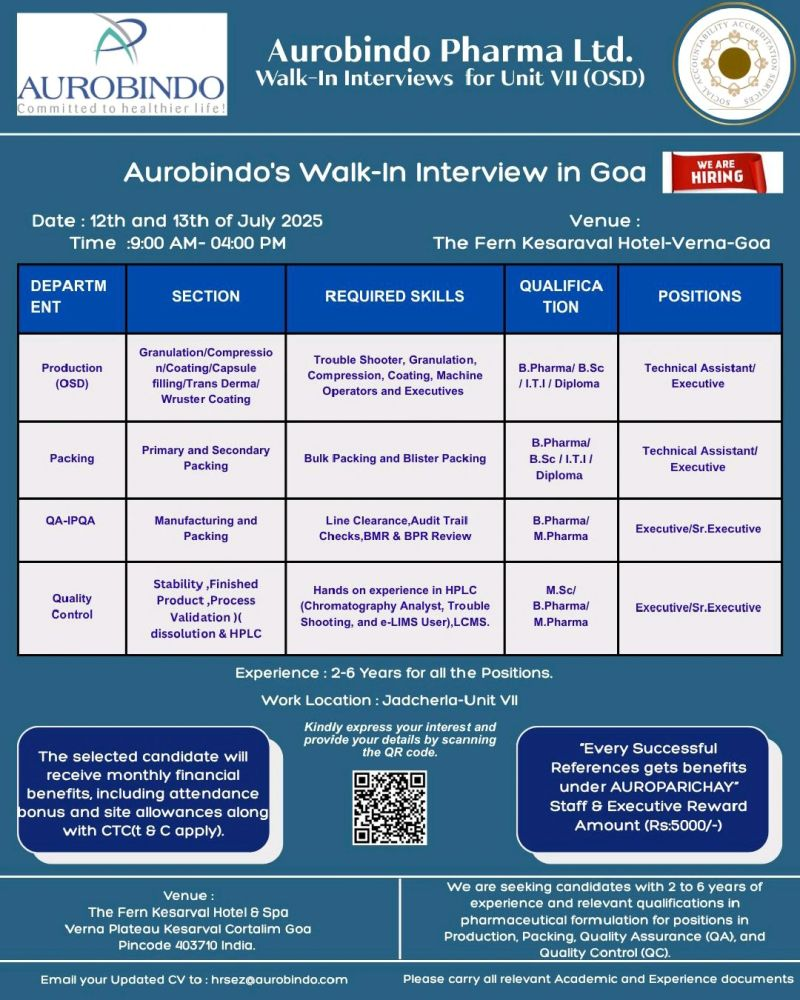
- Dates: Saturday, July 12, 2025, and Sunday, July 13, 2025
- Time: 9:00 AM to 4:00 PM
- Venue: The Fern Kesarval Hotel & Spa, Verna Plateau, Kesarval, Cortalim, Goa – 403710
- Work Location: Aurobindo Pharma Ltd., Unit VII, SEZ, TSIIC, Polepally Village, Jadcherla, Mahbubnagar, Telangana – 509301
- Pre-Registration: Scan the QR code provided in the original job posting to register and express interest.
- Contact: Email resumes to hrsez@aurobindo.com
Required Documents:
- Updated resume (2 copies)
- Original and photocopies of educational certificates (B.Pharm/M.Pharm/B.Sc/M.Sc/ITI/Diploma)
- Last 3 months’ payslips and latest increment letter
- Relieving and service certificates from previous organizations
- Aadhar card and PAN card
- Passport-size photographs (2)
Note:
- Candidates must have 2–6 years of experience in OSD formulations and exposure to USFDA/EU-GMP approved plants.
- Candidates interviewed at Aurobindo in the last 6 months are ineligible.
- Willingness to work in shift operations is required.
- Spot offers may be provided subject to document verification.
- Successful referrals under the AuroParichay program earn a ₹5,000 reward (T&C apply).
- Selected candidates will receive monthly financial benefits, including attendance bonus and site allowances (T&C apply).
Open Positions
Production (OSD)
- Section: Granulation / Compression / Coating / Capsule Filling / Transdermal / Wurster Coating
- Designation: Technical Assistant / Executive
- Qualification: B.Pharm / B.Sc / ITI / Diploma
- Experience: 2–6 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Operate and troubleshoot granulation, compression, coating, capsule filling, transdermal, and Wurster coating equipment.
- Maintain cGMP compliance and adhere to SOPs.
- Handle machine operations, changeovers, and daily reports.
- Support regulatory audits (USFDA, EU-GMP).
Key Skills:
- Expertise in OSD equipment operation and troubleshooting.
- Knowledge of cGMP, SOPs, and regulatory compliance.
Packing
- Section: Primary and Secondary Packing
- Designation: Technical Assistant / Executive
- Qualification: B.Pharm / B.Sc / ITI / Diploma
- Experience: 2–6 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Manage bulk packing and blister packing operations.
- Ensure cGMP compliance and accurate documentation.
- Perform line setup, cleaning, and changeovers.
- Support regulatory audits and batch release processes.
Key Skills:
- Experience in primary/secondary packing and cGMP.
- Familiarity with packing line operations and regulatory standards.
Quality Assurance (QA – IPQA)
- Section: Manufacturing and Packing
- Designation: Executive / Senior Executive
- Qualification: B.Pharm / M.Pharm
- Experience: 2–6 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Conduct in-process quality checks, line clearance, and audit trail checks.
- Review BMR/BPR and manage QMS activities (deviations, CAPA, change control).
- Ensure compliance with cGMP, GDP, and regulatory standards (USFDA, EU-GMP).
- Support audit preparation and execution.
Key Skills:
- Expertise in IPQA, QMS, and regulatory documentation.
- Strong coordination with production and packing teams.
Quality Control (QC)
- Section: Stability, Finished Product, Process Validation, Dissolution & HPLC
- Designation: Executive / Senior Executive
- Qualification: M.Sc / B.Pharm / M.Pharm
- Experience: 2–6 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Perform HPLC (mandatory), LCMS, dissolution, and other analytical tests for stability, finished product, and process validation samples.
- Troubleshoot analytical instruments and use e-LIMS for data management.
- Conduct method validation and ensure GLP compliance.
- Support regulatory audits (USFDA, EU-GMP).
Key Skills:
- Proficiency in HPLC, LCMS, and e-LIMS.
- Knowledge of cGMP, GLP, and regulatory compliance.
Why Join Aurobindo Pharma?
- Global Leader: Contribute to a company with a $3.2 billion market cap, exporting to 150+ countries and manufacturing 500+ products, including OSDs and APIs.
- Regulatory Excellence: Work in a USFDA, EU-GMP, and WHO-GMP compliant facility, with 200+ ANDAs filed and 15 global regulatory approvals.
- Employee Benefits: Enjoy attendance bonuses, site allowances, and the AuroParichay referral program (₹5,000 reward per successful referral, T&C apply).
- Supportive Culture: Rated 3.9/5 on AmbitionBox for job security and teamwork, though work-life balance is moderate at 3.6/5 due to shift demands.
How to Apply
- Walk-In: Attend the interview on July 12 or 13, 2025, at The Fern Kesarval Hotel & Spa, Verna, Goa, with all required documents. Pre-register using the QR code for faster processing.
- For Those Unable to Attend: Email your updated CV to hrsez@aurobindo.com, mentioning the specific role and department (e.g., “Production Executive – Granulation” or “QC Senior Executive”) in the subject line. Include total experience, current CTC, expected CTC, and notice period.
Note: Candidates with USFDA/EU-GMP approved plant experience are preferred.
About Aurobindo Pharma
Founded in 1986, Aurobindo Pharma Ltd. is a Hyderabad-based global pharmaceutical company with 26 manufacturing facilities, including Unit VII in Jadcherla, a key hub for OSD formulations.
Known for generics, APIs, and biosimilars, Aurobindo employs over 15,000 professionals and generates ₹24,000 crore in revenue (FY 2024). The Jadcherla facility specializes in high-volume OSD production, adhering to stringent regulatory standards. Learn more at www.aurobindo.com.
Important Disclaimer
Aurobindo Pharma Ltd. does not charge any fees for recruitment or authorize agencies to collect payments. Report suspicious job offers to hr@aurobindo.com.
Join Aurobindo Pharma and contribute to a “Committed to Healthier Life” at our Jadcherla Unit VII!


