Discover pharma jobs at Avantika Medex! Attend walk-in for QA jobs, production roles in pharmaceutical careers in India. WHO-GMP certified—immediate joiners preferred.
Contents
About the Company
Avantika Medex Pvt. Ltd., established in 2007 by visionary leaders Mr. Lal Babu Singh and Mrs. Vibha Rani Singh, is a premier WHO-GMP certified pharmaceutical manufacturer based in Ahmedabad, Gujarat.
Specializing in high-quality generics like omeprazole capsules and avantovir tablets, the company excels in innovation through advanced formulations and client-centric R&D.
With a strong global presence, including leadership in the Russian market, Avantika ensures regulatory compliance and drives sustainable growth, exporting medicines to foster accessible healthcare worldwide.
Job Details
- Company Name: Avantika Medex Pvt. Ltd.
- Experience: 1 to 10 Years (varies by role)
- Qualification: B.Pharm/M.Pharm/B.Sc/M.Sc/12th/ITI
- Location: J8 to J11, Gallops Industrial Park – 2, National Highway 8A, Village: Vasna Chacharwadi, Tal – Sanand, Ahmedabad-382213, Gujarat, India
- Work Type: On-site
Job Description
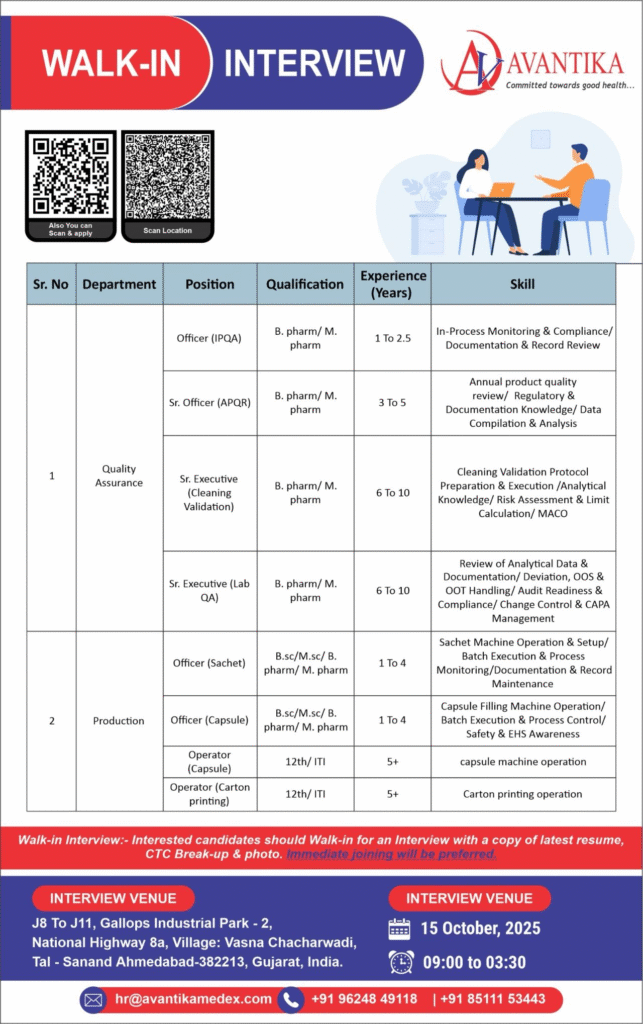
Avantika Medex is hosting an exclusive walk-in drive to strengthen its quality assurance and production teams in Ahmedabad. These pharma jobs target skilled professionals for in-process monitoring, validation, and manufacturing operations. Seize opportunities in pharmaceutical careers in India at a WHO-GMP facility focused on excellence.
Officer (IPQA)
- Department: Quality Assurance
- Role: In-Process Monitoring & Compliance
- Experience: 1 to 2.5 Years
- Education/Qualification: B.Pharm/M.Pharm
Sr. Officer (APQR)
- Department: Quality Assurance
- Role: Annual Product Quality Review
- Experience: 3 to 5 Years
- Education/Qualification: B.Pharm/M.Pharm
Sr. Executive (Cleaning Validation)
- Department: Quality Assurance
- Role: Cleaning Validation Protocol Execution
- Experience: 6 to 10 Years
- Education/Qualification: B.Pharm/M.Pharm
Sr. Executive (Lab QA)
- Department: Quality Assurance
- Role: Analytical Data Review & OOS Handling
- Experience: 6 to 10 Years
- Education/Qualification: B.Pharm/M.Pharm
Officer (Sachet)
- Department: Production
- Role: Sachet Machine Operation & Batch Execution
- Experience: 1 to 4 Years
- Education/Qualification: B.Sc/M.Sc/B.Pharm/M.Pharm
Officer (Capsule)
- Department: Production
- Role: Capsule Filling Machine Operation
- Experience: 1 to 4 Years
- Education/Qualification: B.Sc/M.Sc/B.Pharm/M.Pharm
Operator (Capsule)
- Department: Production
- Role: Capsule Machine Operation & Process Control
- Experience: 5+ Years
- Education/Qualification: 12th/ITI
Operator (Carton Printing)
- Department: Production
- Role: Carton Printing Operation
- Experience: 5+ Years
- Education/Qualification: 12th/ITI
Skills/Qualifications
- In-process monitoring and GMP compliance knowledge
- Expertise in APQR, data analysis, regulatory documentation
- Proficiency in cleaning validation protocols and MACO calculations
- Analytical data review, deviation, OOS/OOT management
- Sachet/capsule machine setup and batch execution
- Safety, EHS awareness, and process control for operators
Key Responsibilities
- Monitor in-process compliance daily
- Compile APQR data for quality reviews
- Execute cleaning validation protocols
- Review analytical data for audit readiness
- Operate sachet machines per BMR
- Fill capsules with process controls
Benefits/Perks
- Immediate joining for fast-track integration
- Career advancement in WHO-GMP environment
- Skill-building in regulatory compliance
- Supportive culture promoting innovation
- Global export exposure opportunities
How to Apply
Walk-in with your latest resume, CTC break-up, and photo for immediate interviews. Email CVs to hr@avantikamedex.com with subject “[Position] Application – Avantika Medex“.
For pharma jobs strategies, explore Pharma Recruiter. Act now—secure your pharmaceutical careers in India!
Walk-in Interview Details
- Date: 15 October 2025
- Time: 09:00 AM to 03:30 PM
- Venue: J8 to J11, Gallops Industrial Park – 2, National Highway 8A, Village: Vasna Chacharwadi, Tal – Sanand, Ahmedabad-382213, Gujarat, India
- Contact/Email: +91 96248 49118 / +91 85111 53443; Email: hr@avantikamedex.com
Why You Should Join
Avantika Medex embodies a culture of dedication to good health and employee recognition, with WHO-GMP certification ensuring stability in pharma jobs since 2007. Experience long-term growth through R&D in generics and international exports, in a compliance-rich environment that values innovation and immediate contributions to affordable healthcare.
FAQs
What qualifications fit QA pharma jobs at Avantika?
B.Pharm/M.Pharm required for officer/executive roles, with 1-10 years experience in validation and compliance.
How to prepare for the walk-in interview?
Bring resume, CTC details, and photo; immediate joiners prioritized for production and QA positions.
Is the role on-site in Ahmedabad?
Yes, all pharma jobs are on-site at the Sanand facility, supporting manufacturing excellence.
What growth exists in production roles?
From operator to officer, with training in GMP and opportunities in global pharmaceutical careers in India.


