Unlock pharma jobs in Gujarat! Aykon Biosciences seeks 8 professionals for Quality Control, Assurance, Production, and Regulatory Affairs in Kheda. 1-2 years exp ideal for sterile injectables manufacturing. Apply now for rewarding pharmaceutical careers!
About the Company
Aykon Biosciences Pvt. Ltd., established in 2021, is a dynamic GMP-certified pharmaceutical manufacturer specializing in sterile liquid injectables (SVP) for ampoules and pre-filled syringes (PFS). Based in Kathlal, Kheda, Gujarat, the company boasts a diverse portfolio exceeding 100 products, including analgesics, NSAIDs, corticosteroids, vitamins, and antimalarials, serving global healthcare needs.
With a commitment to regulatory excellence, innovation in manufacturing processes, and rapid expansion—highlighted by participation in CPHI 2025—Aykon drives quality-driven growth, ensuring compliance with stringent standards while fostering a collaborative environment for breakthrough injectable therapies.
Job Details
- Company Name: Aykon Biosciences Pvt. Ltd.
- Experience: 1-2 years (varies by role)
- Location: Kheda, Gujarat, India
- Work Type: On-site
Job Description
Aykon Biosciences is ramping up its operations in sterile injectable production, creating opportunities across quality and regulatory functions. These roles ensure seamless compliance and efficiency in manufacturing high-impact pharmaceuticals.
Join a forward-thinking team at the forefront of Gujarat’s pharma boom, contributing to global health solutions.
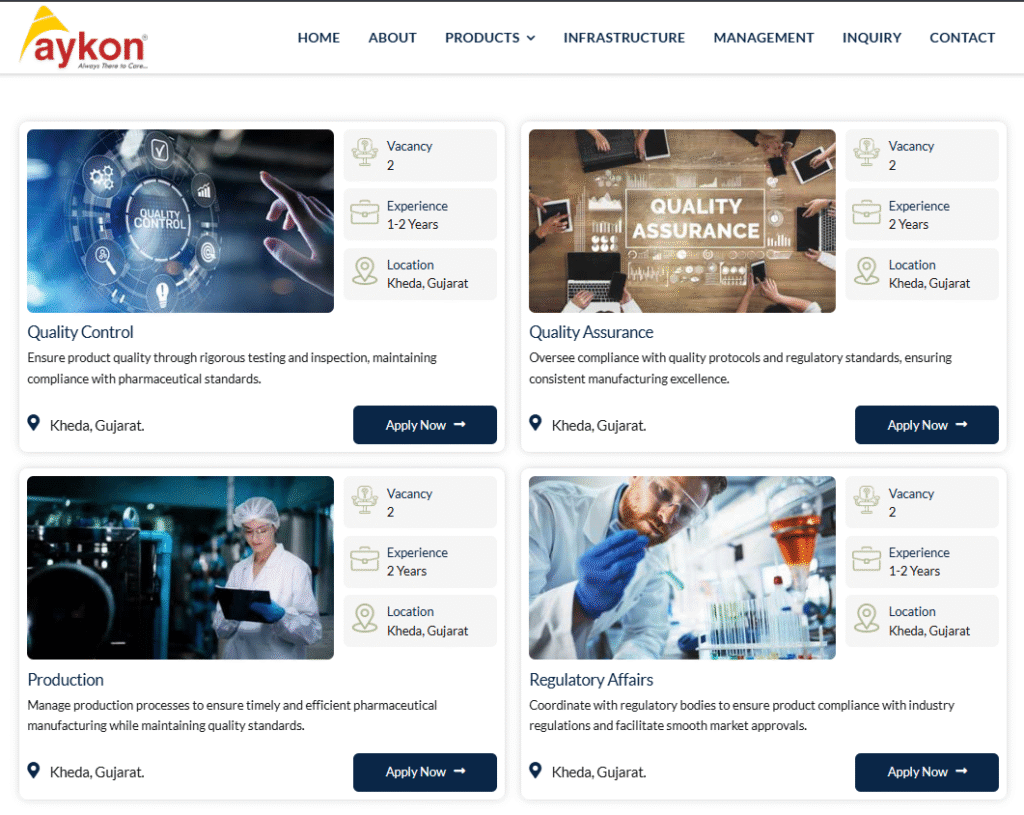
Quality Control
- Department: Quality Control
- Market: Global sterile injectables pharmaceutical industry
- Role: Ensure product quality via testing, inspection, and pharma standards compliance
- Experience: 1-2 years
Quality Assurance
- Department: Quality Assurance
- Market: Global sterile injectables pharmaceutical industry
- Role: Oversee quality protocols and regulatory standards for manufacturing excellence
- Experience: 2 years
Production
- Department: Production
- Market: Global sterile injectables pharmaceutical industry
- Role: Manage processes for timely, efficient pharma manufacturing with quality focus
- Experience: 2 years
Regulatory Affairs
- Department: Regulatory Affairs
- Market: Global sterile injectables pharmaceutical industry
- Role: Coordinate with bodies for compliance and smooth market approvals
- Experience: 1-2 years
Skills/Qualifications
- Solid understanding of GMP and pharmaceutical quality standards
- Experience in testing, inspection, or manufacturing processes for injectables
- Knowledge of regulatory protocols and compliance documentation
- Proficiency in quality assurance tools and production management
- Strong analytical skills for audits and protocol adherence
- Excellent attention to detail and problem-solving abilities
- Team collaboration for cross-functional excellence
- Basic familiarity with pharma industry regulations
Key Responsibilities
- Conduct rigorous product testing and inspections
- Maintain compliance with GMP standards daily
- Oversee quality protocols in manufacturing
- Manage production timelines and efficiency
- Coordinate regulatory submissions and approvals
- Review documentation for audit readiness
- Support team in quality improvement initiatives
Benefits/Perks
- Professional growth via mentorship and challenging projects
- Continuous learning through workshops and training sessions
- Career path planning with performance-based advancement
- Leadership development and cross-functional rotations
- Rewards like bonuses for skill enhancement
- Support for conferences and industry events
- Work-life balance with flexible arrangements
- Inclusive culture boosting engagement and innovation
How to Apply
Visit Aykon Biosciences’ career page at https://aykon.in/career/ and click the “Apply Now” button under your preferred vacancy to submit your resume and details. Tailor your application to highlight relevant pharma experience.
Verified Post
Verification: To confirm the legitimacy of this posting, you can view the original announcement on the Aykon Career page.
For more pharma jobs and Gujarat-specific opportunities, explore Pharma Recruiter. Seize these openings—apply today and propel your career in sterile injectables!
Why You Should Join
Aykon Biosciences embodies an innovative, collaborative culture that sparks curiosity and teamwork, earning recognition through employee-driven ideas and positive reinforcement. Secure enduring career stability in a burgeoning pharma manufacturer expanding its injectable portfolio amid Gujarat’s industrial surge.
Dive into rich learning via mentorship, seminars, and rotations, all within a compliant, excellence-focused ecosystem that champions ethical production and global impact. At Aykon, your expertise crafts life-saving therapies and a fulfilling professional journey.
FAQs
What experience levels are needed for Aykon Biosciences pharma jobs?
1-2 years for Quality Control and Regulatory Affairs; 2 years for Quality Assurance and Production.
How do I apply for these Gujarat pharma positions?
Click “Apply Now” on https://aykon.in/career/ for each role. Visit Pharma Recruiter for similar openings.
What benefits does Aykon offer new hires?
Mentorship, training workshops, career advancement, bonuses, and work-life balance support.
What growth opportunities exist at Aykon?
Leadership programs, job rotations, and conference attendance for rapid progression in pharma manufacturing.


