Unlock pharma jobs at Baroque Pharmaceuticals! Join the walk-in on November 9, 2025, for R&D, QA jobs, production roles, and pharmaceutical careers in India.
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Engineering – Officer
- 3.2 ADL Research Scientist / Sr. Research Scientist
- 3.3 ADL Associate / Research Scientist
- 3.4 F&D – Research Scientist / Sr. Research Scientist
- 3.5 F&D – Associate / Research Scientist
- 3.6 QA – Manager
- 3.7 Production Officer/Sr. Officer
- 3.8 Packing Officer/Sr. Officer
- 3.9 PPIC Officer/Sr. Officer
- 3.10 Operational Excellence Officer/Sr. Officer
- 3.11 Regulatory Affairs Officer/Sr. Officer
- 3.12 Regulatory Affairs Sr. Executive/Ass. Manager
- 3.13 International Business Development – Sr. Executive
- 3.14 International Business Development – Manager
- 3.15 Boiler Operator 2nd Class
- 3.16 R.O. Plant Operator
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
Baroque Pharmaceuticals Private Limited, established in 1994 in Anand, Gujarat, is a pioneering Indian pharmaceutical firm with over three decades of expertise in developing and manufacturing oral solid dosage (OSD) and liquid forms. Led by Chairman and Managing Director Mr. JD Patel, the company emphasizes innovation, quality, and affordability, serving global markets with a broad portfolio of cost-effective medicines.
Holding WHO-GMP certifications and a state-of-the-art R&D facility, Baroque ensures regulatory compliance and sustainable growth, making it a credible leader in pharmaceutical careers in India with international reach.
Job Details
- Company Name: Baroque Pharmaceuticals Pvt. Ltd.
- Experience: Varies by role (1-15 years)
- Qualification: B.E. (Mechanical/Electrical), M.Pharm/B.Pharm/M.Sc./B.Sc., BCA/BCOM/MCOM/MBA, ITI/Relevant certificates
- Location: Kanthariya-Anand / Khambhat, Gujarat, India
- Work Type: On-site
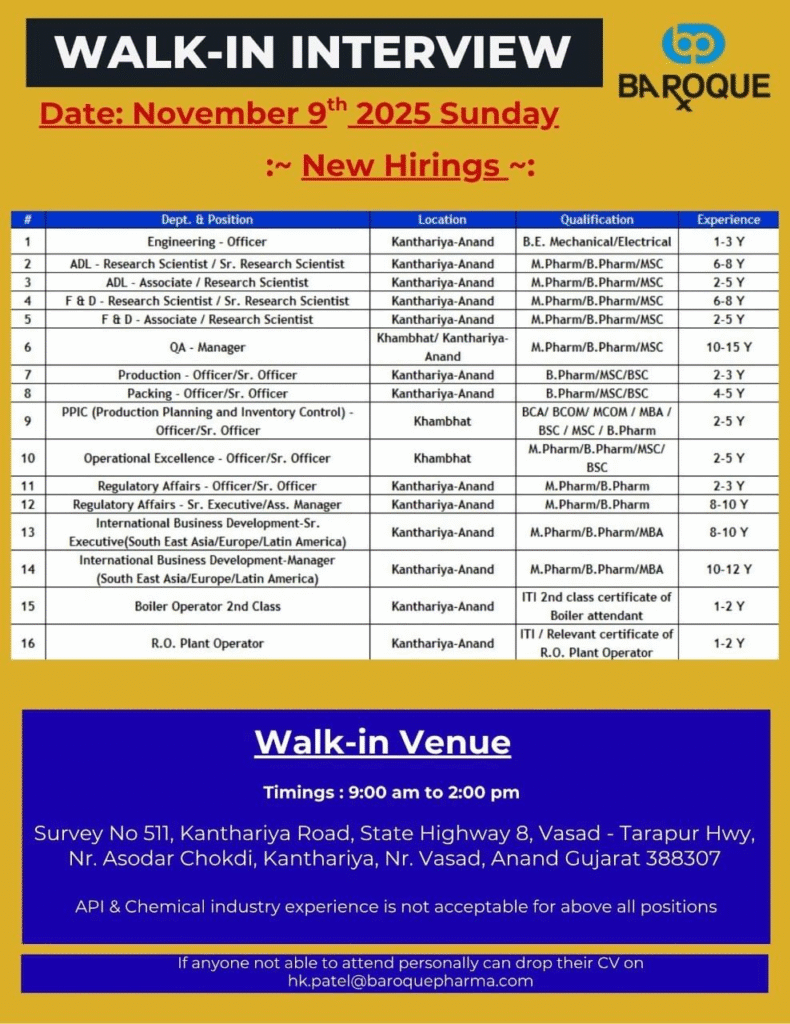
Job Description
Baroque Pharmaceuticals is fueling its expansion with urgent walk-in hiring across R&D, quality, production, and business functions at its Gujarat facilities. This drive targets OSD-experienced talent excluding API/chemical backgrounds to drive innovation and compliance. Diverse pharma jobs await in regulatory affairs, international development, and operational excellence for immediate contributions.
Engineering – Officer
- Department: Engineering
- Role: Officer
- Experience: 1-3 Years
- Education/Qualification: B.E. Mechanical/Electrical
ADL Research Scientist / Sr. Research Scientist
- Department: ADL
- Role: Research Scientist / Sr. Research Scientist
- Experience: 6-8 Years
- Education/Qualification: M.Pharm/B.Pharm/M.Sc.
ADL Associate / Research Scientist
- Department: ADL
- Role: Associate / Research Scientist
- Experience: 2-5 Years
- Education/Qualification: M.Pharm/B.Pharm/M.Sc.
F&D – Research Scientist / Sr. Research Scientist
- Department: Formulation & Development
- Role: Research Scientist / Sr. Research Scientist
- Experience: 6-8 Years
- Education/Qualification: M.Pharm/B.Pharm/M.Sc.
F&D – Associate / Research Scientist
- Department: Formulation & Development
- Role: Associate / Research Scientist
- Experience: 2-5 Years
- Education/Qualification: M.Pharm/B.Pharm/M.Sc.
QA – Manager
- Department: Quality Assurance
- Role: Manager
- Experience: 10-15 Years
- Education/Qualification: M.Pharm/B.Pharm/M.Sc.
Production Officer/Sr. Officer
- Department: Production
- Role: Officer/Sr. Officer
- Experience: 2-3 Years
- Education/Qualification: B.Pharm/M.Sc./B.Sc.
Packing Officer/Sr. Officer
- Department: Packing
- Role: Officer/Sr. Officer
- Experience: 4-5 Years
- Education/Qualification: B.Pharm/M.Sc./B.Sc.
PPIC Officer/Sr. Officer
- Department: Production Planning and Inventory Control
- Role: Officer/Sr. Officer
- Experience: 2-5 Years
- Education/Qualification: BCA/BCOM/MCOM/MBA/B.Sc./M.Sc./B.Pharm
Operational Excellence Officer/Sr. Officer
- Department: Operational Excellence
- Role: Officer/Sr. Officer
- Experience: 2-3 Years
- Education/Qualification: M.Pharm/B.Pharm/M.Sc./B.Sc.
Regulatory Affairs Officer/Sr. Officer
- Department: Regulatory Affairs
- Role: Officer/Sr. Officer
- Experience: 8-10 Years
- Education/Qualification: M.Pharm/B.Pharm
Regulatory Affairs Sr. Executive/Ass. Manager
- Department: Regulatory Affairs
- Role: Sr. Executive/Ass. Manager
- Experience: 2-5 Years? (Text has 2-5 Y under 9, but seems misaligned; assuming based on context)
- Education/Qualification: M.Pharm/B.Pharm
International Business Development – Sr. Executive
- Department: International Business Development
- Role: Sr. Executive (South East Asia/Europe/Latin America)
- Experience: 8-10 Years
- Education/Qualification: M.Pharm/B.Pharm/MBA
International Business Development – Manager
- Department: International Business Development
- Role: Manager (South East Asia/Europe/Latin America)
- Experience: 10-12 Years
- Education/Qualification: M.Pharm/B.Pharm/MBA
Boiler Operator 2nd Class
- Department: Operations
- Role: Boiler Operator 2nd Class
- Experience: 1-2 Years
- Education/Qualification: ITI 2nd class certificate of Boiler attendant
R.O. Plant Operator
- Department: Operations
- Role: R.O. Plant Operator
- Experience: 1-2 Years
- Education/Qualification: ITI/Relevant certificate of R.O. Plant Operator
Skills/Qualifications
- Advanced knowledge in OSD formulation and analytical development
- Expertise in QA management and GMP compliance for regulatory audits
- Proficiency in production planning, inventory control, and operational optimization
- Strong regulatory affairs skills for dossier preparation and international filings
- Business development acumen for South East Asia, Europe, and Latin America markets
- Engineering troubleshooting in mechanical/electrical systems for pharma plants
- Hands-on operation of boilers and RO plants with safety certifications
Key Responsibilities
- Develop and validate formulations in F&D/ADL labs
- Lead QA teams in audit preparations and compliance reviews
- Oversee production and packing operations for efficiency
- Manage inventory and planning in PPIC for seamless supply
- Drive regulatory submissions and query resolutions
- Expand business partnerships in target international regions
- Maintain engineering equipment and utility operations
Benefits/Perks
- Career progression in a WHO-GMP certified innovator
- Ongoing training in R&D and regulatory best practices
- Attractive salary with performance-linked incentives
- Supportive culture focused on work-life harmony
- International exposure via global business initiatives
How to Apply
Candidates unable to attend the walk-in can email their CV to hk.patel@baroquepharma.com, specifying the preferred role. Include a brief cover note on your OSD experience. For broader pharmaceutical careers in India, visit Pharma Recruiter.
Note: API/chemical industry experience not considered. Act fast—secure your spot in Baroque’s growth story today!
Walk-in Interview Details
- Date: November 9th, 2025 (Sunday)
- Time: 9:00 AM to 2:00 PM
- Venue: Survey No 511, Kanthariya Road, State Highway 8, Vasad – Tarapur Hwy, Nr. Asodar Chokdi, Kanthariya, Nr. Vasad, Anand, Gujarat 388307
- Contact/Email: hk.patel@baroquepharma.com
Why You Should Join
Baroque Pharmaceuticals embodies a culture of excellence and innovation, rated highly for employee development in Gujarat’s pharma hub. With stable expansion and global ambitions, it promises long-term career security in a compliant, forward-thinking environment.
Seize learning opportunities in OSD R&D, regulatory strategies, and market expansion, propelling your pharmaceutical careers in India while delivering impactful, affordable healthcare solutions worldwide.
FAQs
Who qualifies for these pharma jobs at Baroque?
OSD-experienced professionals with 1-15 years; M.Pharm/B.Pharm/M.Sc./B.E./ITI qualifications. No API/chemical backgrounds.
How to apply if I can’t make the walk-in?
Email your CV to hk.patel@baroquepharma.com with role details for prompt review and potential interviews.
What to expect at the November 9 walk-in?
Technical discussions on OSD processes, regulatory knowledge, and experience proofs. Bring resume and certificates.
What growth paths exist in QA jobs or production jobs?
Mentorship programs, international rotations, and promotions in a GMP-focused firm, with competitive pay for top talent.


