BDR Pharmaceuticals International Pvt. Ltd., a leader in niche pharmaceutical manufacturing, is hosting a Walk-in Drive on June 21, 2025, at our USFDA-approved facility in Baska, Gujarat. We’re hiring for multiple roles in Warehouse, Quality Control (QC), Production, and Manufacturing, focusing on injectables and oral solid dosage (OSD) forms. Join our mission to make affordable, life-saving medicines accessible globally.
Contents
About BDR Pharmaceuticals
Established in 2002, BDR is a Great Place to Work® certified company specializing in Oncology, Critical Care, Gynecology, and Neurology. Our Baska plant is WHO-GMP and EU-GMP compliant, with plans for further accreditations like ANVISA. We excel in early molecule identification, innovative drug delivery systems, and multi-branding strategies to ensure affordable healthcare. Learn more at BDR Pharmaceuticals.
Walk-in Drive Details
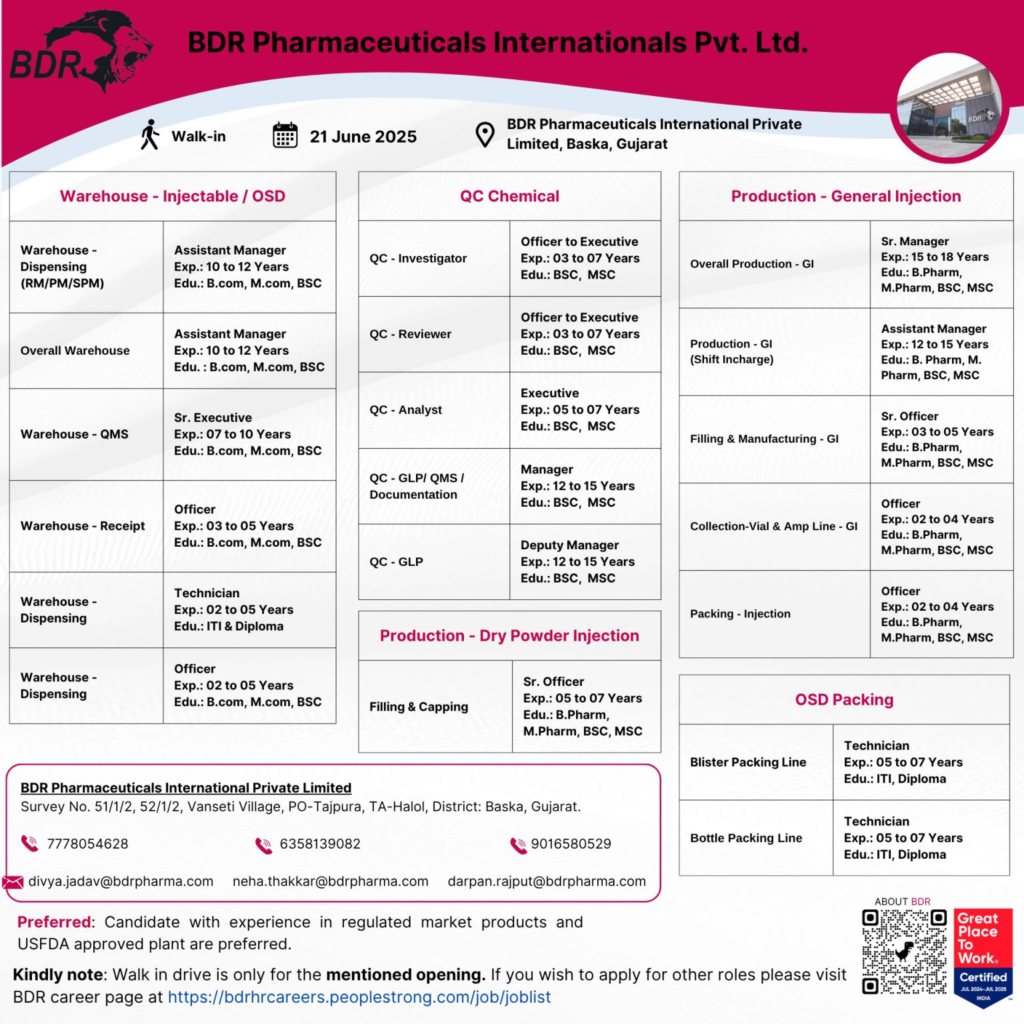
We’re seeking candidates with experience in regulated market products and USFDA-approved plants for our Baska facility. Below are the event details:
| Event Details | Information |
|---|---|
| Date | June 21, 2025 (Saturday) |
| Time | 9:00 AM to 5:00 PM IST (assumed based on prior events) |
| Venue | BDR Pharmaceuticals International Pvt. Ltd., Survey No. 51/1/2, 52/1/2, Vanseti Village, PO-Tajpura, TA-Halol, District: Baska, Gujarat |
| Contact Numbers | 7778054628, 6358139082, 9016580529 |
| Contact Emails | divya.jadav@bdrpharma.com, neha.thakkar@bdrpharma.com, darpan.rajput@bdrpharma.com |
| Application Note | For other roles, visit BDR Careers |
Job Opportunities
Below are the open positions, qualifications, experience, and responsibilities. All roles are based at the Baska, Gujarat facility and prioritize candidates with USFDA-approved plant experience.
1. Warehouse – Injectable / OSD
Positions & Details:
- Assistant Manager – Overall Warehouse:
- Experience: 10–12 years
- Qualification: B.Com, M.Com, B.Sc.
- Responsibilities: Oversee warehouse operations, manage QMS, handle SAP for inventory, and ensure compliance for RM/PM/SPM.
- Assistant Manager – Dispensing (RM/PM/SPM):
- Experience: 10–12 years
- Qualification: B.Com, M.Com, B.Sc.
- Responsibilities: Manage dispensing of raw materials (RM), packaging materials (PM), and semi-finished products (SPM).
- Officer – Receipt:
- Experience: 3–5 years
- Qualification: B.Com, M.Com, B.Sc.
- Responsibilities: Handle material receipt and documentation.
- Technician – Receipt:
- Experience: 2–5 years
- Qualification: ITI, Diploma
- Responsibilities: Support material receipt and inventory tracking.
- Officer – Dispensing:
- Experience: 2–5 years
- Qualification: B.Com, M.Com, B.Sc.
- Responsibilities: Execute dispensing processes for RM/PM/SPM.
2. Quality Control (QC) – Chemical
Positions & Details:
- Officer to Executive – QC Investigator:
- Experience: 3–7 years
- Qualification: B.Sc., M.Sc.
- Responsibilities: Investigate OOS/OOT, perform chemical analysis of RM/IP/FP, and support HPLC/GC testing.
- Sr. Executive – QC Analyst:
- Experience: Not specified (assumed 5–8 years based on prior postings)
- Qualification: B.Sc., M.Sc.
- Responsibilities: Conduct HPLC, GC, and water analysis for product quality.
- Sr. Executive – QC Reviewer:
- Experience: Not specified (assumed 5–8 years)
- Qualification: B.Sc., M.Sc.
- Responsibilities: Review analytical data and ensure compliance with GLP/QMS.
- Manager – QC-GLP/QMS/Documentation:
- Experience: 12–15 years
- Qualification: B.Sc., M.Sc.
- Responsibilities: Oversee GLP, QMS, and documentation for regulatory audits.
- Deputy Manager – QC-GLP:
- Experience: 12–15 years
- Qualification: B.Sc., M.Sc.
- Responsibilities: Manage GLP processes and ensure audit readiness.
3. Production – General Injection (GI)
Positions & Details:
- Officer to Executive – Overall Production:
- Experience: 3–7 years
- Qualification: B.Sc., M.Sc.
- Responsibilities: Manage aseptic manufacturing, vial/ampoule filling, and autoclave operations.
- Executive – Shift Incharge:
- Experience: 5–7 years
- Qualification: B.Sc., M.Sc.
- Responsibilities: Lead shift operations, ensure QMS, and manage BMR.
- Sr. Manager – Filling & Manufacturing:
- Experience: 15–18 years
- Qualification: B.Pharm, M.Pharm, B.Sc., M.Sc.
- Responsibilities: Oversee vial/ampoule filling, aseptic areas, and production compliance.
- Assistant Manager – Filling & Manufacturing:
- Experience: 12–15 years
- Qualification: B.Pharm, M.Pharm, B.Sc., M.Sc.
- Responsibilities: Manage filling and manufacturing processes for injectables.
- Sr. Officer – Collection (Vial & Amp Line):
- Experience: 3–5 years
- Qualification: B.Pharm, M.Pharm, B.Sc., M.Sc.
- Responsibilities: Handle vial/ampoule collection and ensure quality.
- Officer – Filling & Capping:
- Experience: 2–4 years
- Qualification: B.Pharm, M.Pharm, B.Sc., M.Sc.
- Responsibilities: Operate filling and capping equipment in aseptic areas.
4. Production – Dry Powder Injection (DPI)
- Position: Officer
- Experience: 2–4 years
- Qualification: B.Pharm, M.Pharm, B.Sc., M.Sc.
- Responsibilities: Manage DPI filling, autoclave, and aseptic operations.
- Skills: Knowledge of DPI manufacturing and sterilization processes.
5. Packing – Injection / OSD
Positions & Details:
- Officer – Packing Injection:
- Experience: 2–4 years
- Qualification: B.Pharm, M.Pharm, B.Sc., M.Sc.
- Responsibilities: Oversee injection packing (vials/ampoules).
- Technician – Blister Packing Line:
- Experience: 5–7 years
- Qualification: ITI, Diploma
- Responsibilities: Operate blister packing equipment for OSD.
- Technician – Bottle Packing Line:
- Experience: 5–7 years
- Qualification: ITI, Diploma
- Responsibilities: Manage bottle packing for OSD.
- Technician – OSD Packing:
- Experience: 5–7 years
- Qualification: ITI, Diploma
- Responsibilities: Handle OSD packing processes.
Why Join BDR Pharmaceuticals?
BDR is a niche player in global pharmaceuticals, recognized for innovation and affordability. Benefits include:
- Competitive Salaries: Sr. Manager roles average ₹15–20 LPA, Officers ₹3–6 LPA in Gujarat.
- Global Exposure: Work in a USFDA-approved, WHO-GMP/EU-GMP facility.
- Career Growth: Training in QMS, HPLC, and aseptic manufacturing.
- Innovative Culture: Contribute to novel drug delivery systems and biosimilars.
- Supportive Environment: Rated 4.2/5 for job security and culture.
- Note: No Saturday offs; fast-paced work environment.
Why These Roles Matter
These roles support BDR’s production of critical injectables and OSDs in Oncology and Critical Care. From QC analysis to aseptic filling, you’ll ensure high-quality, affordable medicines reach patients globally. Baska’s facility, with 2,500+ pharma jobs in the region, is a hub for innovation.
Growth Opportunities
BDR offers training in USFDA compliance, process validation, and SAP. The Baska plant’s modern infrastructure and DSIR-accredited R&D center provide exposure to global standards. Employees praise skill development but note urgent timelines.
Work Environment
The Baska facility features isolators, scrubber systems, and ISO Class VI conditions, ensuring safety and compliance. Expect a fast-paced, team-oriented culture with supportive staff. Challenges include long hours and limited lunch facilities at nearby Padra.
How to Attend
Join us on June 21, 2025, at the Baska plant. Bring:
- Updated CV.
- Educational certificates.
- Experience letters or payslips.
- Photo ID and passport-size photos.
Alternatively, email your CV to divya.jadav@bdrpharma.com, neha.thakkar@bdrpharma.com, or darpan.rajput@bdrpharma.com with the position title in the subject line. For other roles, apply via BDR Careers.
Preparation Tips
- Highlight USFDA experience and regulated market exposure.
- Showcase skills in HPLC/GC, QMS, or aseptic filling.
- Bring examples of OOS investigations or process optimization.
- Prepare for technical questions (e.g., “What is assay by HPLC?” or “Difference between calibration and validation?”).
Important Disclaimer
BDR Pharmaceuticals maintains a transparent recruitment process. We do not charge fees or use free email services (e.g., Gmail, Yahoo) for job offers. Verify opportunities through official emails or BDR Careers. Report suspicious activities to Ombudsman@bdrpharma.com or call +91-22-40560560.
Stay Safe from Fraud
- Confirm offers through official BDR channels.
- Avoid sharing personal or financial information with unverified sources.
- Contact HR at 7778054628, 6358139082, or 9016580529 for clarifications.
Why Baska, Gujarat?
Baska, near Halol, is a pharmaceutical hub with CGMP-compliant facilities like BDR’s. Its proximity to Vadodara (30 km) offers connectivity and a growing job market with 2,500+ pharma roles. The region’s industrial focus makes it ideal for career growth.
Join BDR’s Mission
BDR Pharmaceuticals is committed to making good health a right, not a luxury. By joining our Baska team, you’ll contribute to affordable, innovative medicines in critical segments. Attend our walk-in drive on June 21, 2025, or email your CV to start your journey.
Next Steps
Arrive early for registration on June 21, 2025. The selection process may include technical interviews and assessments. Selected candidates will receive spot offers post-verification. Check emails for updates from HR contacts.
Contact Us
For queries, email divya.jadav@bdrpharma.com, neha.thakkar@bdrpharma.com, darpan.rajput@bdrpharma.com, or call 7778054628, 6358139082, or 9016580529. Visit BDR Contact for more details.
Advance Your Career
Join BDR Pharmaceuticals to drive innovation in injectables and OSDs. Attend our Walk-in Drive on June 21, 2025, in Baska, Gujarat, and bring your passion for healthcare!


