Join Bharat Parenterals Ltd., a WHO GMP and ISO-certified pharmaceutical company established in 1992 by Mr. Ramesh Desai, dedicated to delivering world-class, affordable medicines.
We are hosting a walk-in drive on Monday, June 2, and Tuesday, June 3, 2025, at our state-of-the-art facility in Vadodara, Gujarat, for roles across Formulation and Development, Finance/Import/Export, QC Microbiology, Maintenance, Project Engineering, General Injection, Beta Lactam, General Production, and Quality Assurance.
With over 800 products and a presence in 35+ countries, Bharat Parenterals offers a dynamic environment for career growth in pharmaceutical jobs.
Contents
Why Choose Bharat Parenterals Ltd?
Bharat Parenterals, headquartered in Vadodara, is a leader in contract manufacturing, specializing in general, β-lactam, and antiretroviral formulations, including tablets, capsules, dry syrups, injectables, and more.
Our 30,000 sq.ft facility, compliant with US FDA, WHO GMP, and ISO standards, supports innovative R&D and robust quality systems. Rated 2.6/5 for work-life balance on AmbitionBox (80+ reviews), we offer competitive salaries and long-term association incentives, fostering a committed workforce.
Open Positions at Vadodara Facility
We are seeking freshers and experienced professionals with pharmaceutical experience for the following roles at our facility in Haripura, Vadodara:
| Department | Position | Qualification | Experience | No. of Posts | Key Skills Required |
|---|---|---|---|---|---|
| Formulation and Development | Officer | M.Pharm | 5+ Years | 1 | Assist in formulation development activities, knowledge of cGMP |
| Finance/Import/Export | Sr. Executive / Assistant Manager | Postgraduate or Import-Export Management | 13 Years | 2 | Export documentation, logistics, freight forwarding, DGFT applications, Advance Authorization/EPCG |
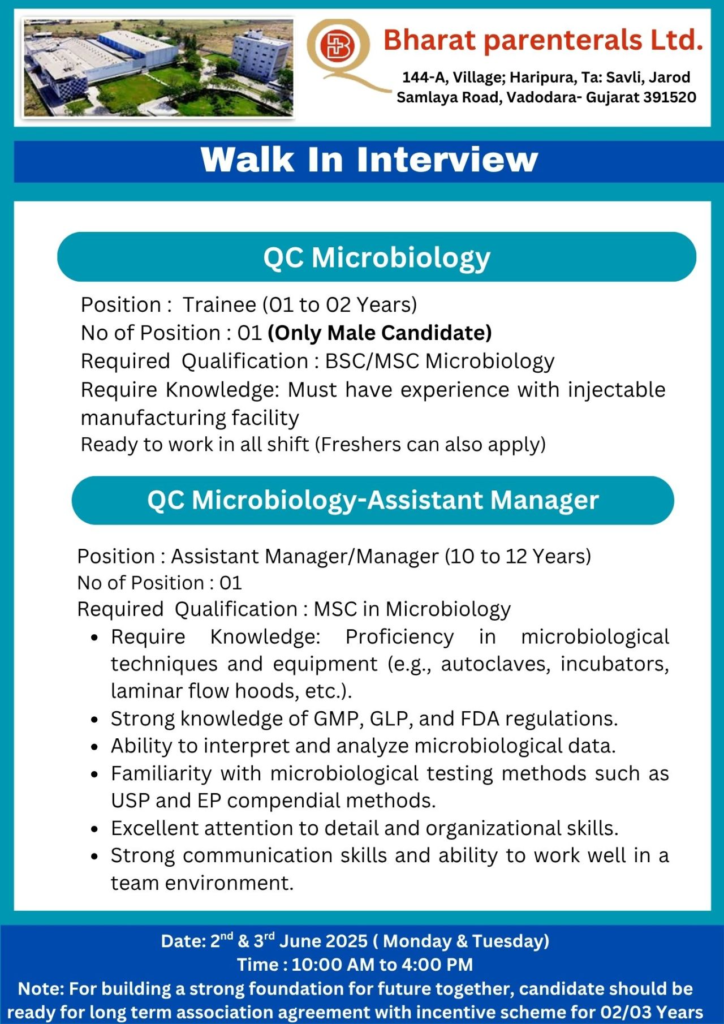
| QC Microbiology | Trainee | B.Sc / M.Sc (Microbiology) | 1-2 Years | 1 (Male) | Experience in injectable manufacturing, shift flexibility (freshers eligible) |
| QC Microbiology | Assistant Manager / Manager | M.Sc (Microbiology) | 10-12 Years | 1 | Microbiological techniques, GMP/GLP, USP/EP testing, data analysis, FDA compliance |
| Maintenance Engineer | Engineer / Sr. Engineer | B.E (Electrical/Maintenance) | 4-6 Years | 1 | Split AC/VRV, PM of equipment, electrical breakdown, solar power system maintenance |
| Maintenance Engineer | Technician (Electrical) | ITI / Diploma (Electrician) | 10 Years | 1 | HVAC, boiler, RO/EDI, WFI plant electrical maintenance, LT panels, motors |
| Project Engineer | Jr. Officer / Officer | Master’s in Project/Engineering Management | 1-3 Years | 1 | Civil/non-civil industrial project management |
| Personal Assistant | Personal Assistant | B.Com / BA (English) | 0-1 Year | 1 | Fluent English communication (freshers eligible) |
| General Injection | Trainee | M.Sc | 1-2 Years | 1 | Injectable packing, ERP knowledge (freshers eligible) |
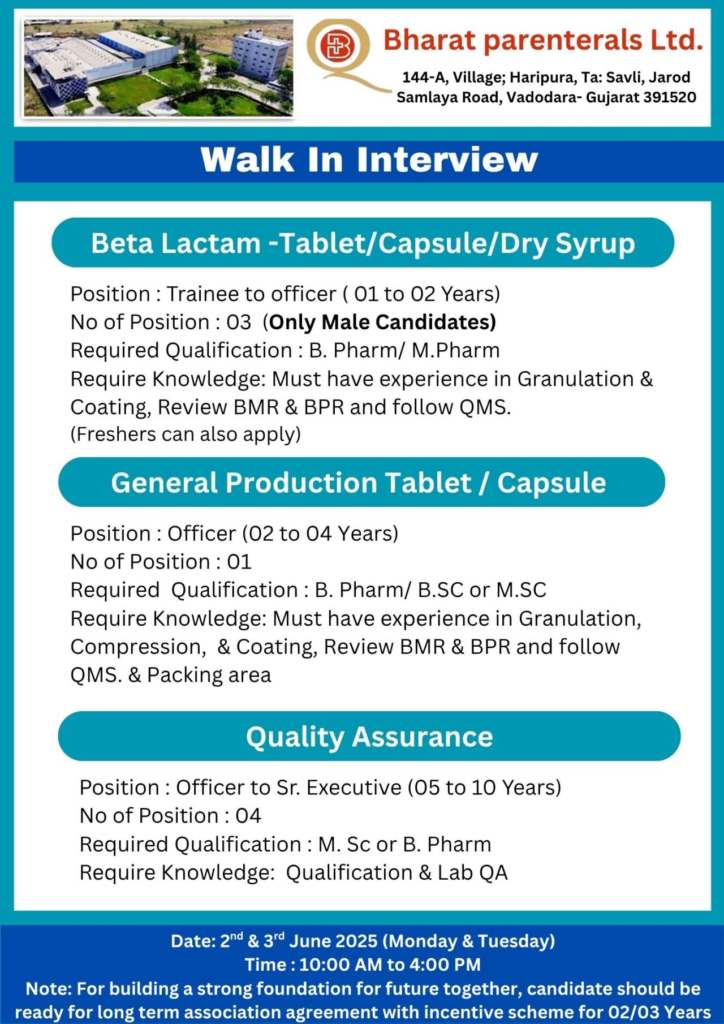
| Beta Lactam (Tablet/Capsule/Dry Syrup) | Trainee to Officer | B.Pharm / M.Pharm | 1-2 Years | 3 (Male) | Granulation, coating, BMR/BPR review, QMS (freshers eligible) |
| General Production (Tablet/Capsule) | Officer | B.Pharm / B.Sc / M.Sc | 2-4 Years | 1 | Granulation, compression, coating, BMR/BPR review, QMS, packing |
| Quality Assurance | Officer to Sr. Executive | M.Sc / B.Pharm | 5-10 Years | 4 | Qualification, lab QA, cGMP compliance |
Job Responsibilities
- Formulation and Development Officer: Support formulation development, conduct trials, and ensure cGMP-compliant documentation.
- Finance/Import/Export Sr. Executive/Assistant Manager: Manage export documentation (invoices, packing lists, AWB/BL drafts), coordinate with freight forwarders/CHAs, handle third-party inspections, and process DGFT applications for Advance Authorization/EPCG.
- QC Microbiology Trainee: Conduct microbial testing and environmental monitoring in injectable facilities, work in rotating shifts.
- QC Microbiology Assistant Manager/Manager: Oversee microbiological testing (USP/EP methods), ensure GMP/GLP compliance, analyze data, and manage equipment like autoclaves and laminar flow hoods.
- Maintenance Engineer (Engineer/Sr. Engineer): Maintain split AC/VRV systems, perform PM on equipment, resolve electrical breakdowns, and manage solar power systems.
- Maintenance Engineer (Technician): Troubleshoot electrical issues in HVAC, boilers, RO/EDI, WFI plants, and maintain LT panels, motors, and lighting.
- Project Engineer (Jr. Officer/Officer): Manage civil/non-civil industrial projects, ensuring timely execution and compliance.
- Personal Assistant: Provide administrative support with fluent English communication.
- General Injection Trainee: Handle injectable packing operations, use ERP systems, and maintain cGMP standards.
- Beta Lactam Trainee to Officer: Perform granulation, coating, and QMS activities; review BMR/BPR for tablets, capsules, and dry syrups.
- General Production Officer: Manage granulation, compression, coating, and packing; ensure QMS and BMR/BPR compliance.
- Quality Assurance Officer to Sr. Executive: Conduct lab QA, equipment qualification, and ensure cGMP compliance.
Desired Candidate Profile
- Qualifications: M.Pharm, B.Pharm, B.Sc, M.Sc, B.E, ITI, Diploma, Postgraduate, or Master’s as specified.
- Experience: 0-13 years, with pharmaceutical experience mandatory for most roles; freshers eligible for select positions.
- Skills:
- Formulation: Expertise in formulation development and cGMP.
- Finance/Import/Export: Export/import regulations, logistics, DGFT processes.
- QC Microbiology: Microbial testing, GMP/GLP, USP/EP methods.
- Maintenance: Electrical/mechanical maintenance, HVAC, solar systems.
- Project: Industrial project management.
- General Injection/Beta Lactam/General Production/QA: Granulation, coating, QMS, BMR/BPR review, injectable packing, lab QA.
- Note: Preference for male candidates in some roles due to operational needs; long-term association (2-3 years) with incentive schemes required.
Walk-In Interview Details
- Date: Monday, June 2, and Tuesday, June 3, 2025
- Time: 10:00 AM to 4:00 PM
- Venue: Bharat Parenterals Ltd., 144-A, Village Haripura, Ta: Savli, Jarod Samlaya Road, Vadodara, Gujarat 391520
- Work Location: Same as venue
Documents to Bring:
- Updated CV
- Passport-sized photo
- Latest salary slip
- Educational certificates
- Experience certificates
- Aadhar and PAN card
How to Apply
Attend the walk-in drive with the required documents. For pre-registration or queries, email your CV to recruitment@bplindia.in with the subject “Application for [Position]” (e.g., QC Microbiology Trainee). Contact +91 990-992-8332 or visit www.bplindia.in for details. Follow Bharat Parenterals on LinkedIn for updates.
Verified by Trusted HRs
The post is released by the Bharat Parenterals Ltd. LinkedIn page. Click here to visit the post



Why Haripura, Vadodara?
Located 24 km from Vadodara, our Haripura facility is a pharmaceutical hub with US FDA-compliant infrastructure for general, β-lactam, and antiretroviral products. Vadodara’s industrial ecosystem offers excellent connectivity and a vibrant professional community, ideal for pharma jobs.
Application Tips
To excel:
- Highlight pharmaceutical experience in injectables, β-lactam, or export/import operations.
- Emphasize cGMP, QMS, or microbiological testing expertise (e.g., USP/EP, HPLC).
- For freshers, showcase academic projects or internships in relevant areas.
- Demonstrate readiness for long-term commitment (2-3 years) with incentive schemes.
Why Join Bharat Parenterals Ltd?
With 23,812+ LinkedIn followers and a legacy of 25+ years, Bharat Parenterals is a fast-growing CDMO with a 30,000 sq.ft facility producing 800+ products. Our accolades include the 2022-23 CSR Healthcare Award, and we reported ₹59.82 crore in net sales (Dec 2023). Despite a 2.6/5 career growth rating, we offer competitive salaries and global exposure across 35+ countries. Join our 200+ strong team to drive innovation in pharmaceutical manufacturing.
Note: Candidates must commit to a 2-3 year association with incentive schemes to build a strong foundation with Bharat Parenterals.
Don’t miss this opportunity to join a leading pharmaceutical company! Attend our walk-in drive on June 2-3, 2025, in Vadodara, and advance your career with Bharat Parenterals Ltd!Show in sidebar


