Exciting pharma jobs in J&K! Walk-in interview on Oct 5, 2025, for injectable facility roles in warehouse, production, QA, QC, and microbiology. Ideal for experienced professionals in pharmaceutical careers.
Contents
About the Company
Biovonic Healthcare Pvt. Ltd., a joint venture between Ravenbhel Healthcare and Leeford, is establishing a green field injectable facility in Kathua, Jammu & Kashmir. Founded to manufacture high-quality sterile injectables, Biovonic emphasizes innovation, regulatory compliance, and GMP standards.
As a rapidly growing player in the pharma sector, it offers opportunities for career advancement in pharmaceutical careers focused on sterile manufacturing.
Job Details
- Company Name: Biovonic Healthcare Pvt. Ltd.
- Experience: 4-15 years in pharma industry (role-specific)
- Qualification: B.Pharm, M.Pharm, B.Sc, M.Sc, M.Com, B.Sc/M.Sc Microbiology
- Location: Ghatti, Kathua, J&K
- Work Type: Full-time
Job Description
Biovonic Healthcare is hosting a walk-in interview for multiple positions in its new injectable facility. These pharma jobs target candidates with injectable experience, focusing on documentation, compliance, and production excellence in a green field setup.
Join pharmaceutical careers in J&K with hands-on roles in sterile manufacturing.
Warehouse
- Department: Warehouse
- Role: Preparation of warehouse SOPs and documentation with strong written English skills.
- Experience: Minimum 10-15 years (Dy. Manager/Manager); 4 years (Officer).
- Education/Qualification: Any Graduate (BSc/D.Pharm/MA/M.Com).
Production (Injectable)
- Department: Production (Injectable)
- Role: Expertise in documentation, review, machine installations for lyophilizers, autoclaves, liquid ampoule/vial lines, and dry powder injectables; strong written English for SOPs, URS, IQ/OQ/PQ.
- Experience: Minimum 10-15 years (Dy. Manager/Manager); 6-8 years (Executive).
- Education/Qualification: M.Pharm/B.Pharm.
Quality Assurance (GMP Documentation)
- Department: Quality Assurance (GMP Documentation)
- Role: Preparation and review of QMS, risk assessment, qualifications, and validations for SVP, lyophilization, dry powder injectables; good written English control.
- Experience: Minimum 10-15 years (Dy. Manager/Manager); 6-8 years (Executive); 4-6 years (Officer).
- Education/Qualification: M.Sc/B.Pharm (Dy. Manager/Manager, Executive); M.Pharm/B.Pharm (Officer).
Quality Control
- Department: Quality Control
- Role: Documentation and qualifications of lab instruments, SOP preparation/review, URS qualification, water system qualification; strong written English.
- Experience: Minimum 10-15 years (Asst/Dy. Manager); 4-6 years (Officer).
- Education/Qualification: M.Sc/M.Pharm/B.Pharm, B.Sc.
Microbiology
- Department: Microbiology
- Role: Documentation and qualifications of lab instruments, SOP preparation/review, EM monitoring, water sampling, sterility, culture/MLT.
- Experience: Minimum 10-15 years (Asst/Dy. Manager); 4-6 years (Officer).
- Education/Qualification: B.Sc/M.Sc Microbiology.
Skills/Qualifications
- Proficiency in GMP documentation, SOP preparation, and review.
- Expertise in qualifications, validations, and risk assessments for injectables.
- Strong written English for technical documents like URS, IQ/OQ/PQ.
- Knowledge of sterile manufacturing processes, including lyophilization and water systems.
- Experience with lab instruments, EM monitoring, and microbiology testing.
- Organizational skills for warehouse and production management.
Key Responsibilities
- Develop and review SOPs, QMS, and documentation for warehouse and production.
- Handle machine installations, process validations for lyophilizers, autoclaves, and filling lines.
- Conduct risk assessments, qualifications, and validations for SVP and dry powder injectables.
- Perform lab instrument qualifications, water system validations, and sterility testing.
- Monitor environmental controls, water sampling, and microbial limit tests.
- Ensure compliance with pharma standards in a green field injectable facility.
Benefits/Perks
- Competitive salary based on experience in pharma jobs.
- Opportunities for leadership roles in a new injectable manufacturing plant.
- Professional growth in a joint venture with established pharma partners.
- Supportive environment focused on innovation and compliance.
- Contribution to high-quality sterile healthcare products.
How to Apply
Submit your CV to pramod.gupta@ravenbhel.com or hr@ravenbhel.com. For queries, call +91-9596837772 or +91-8899333151. Explore more opportunities at Pharma Recruiter.
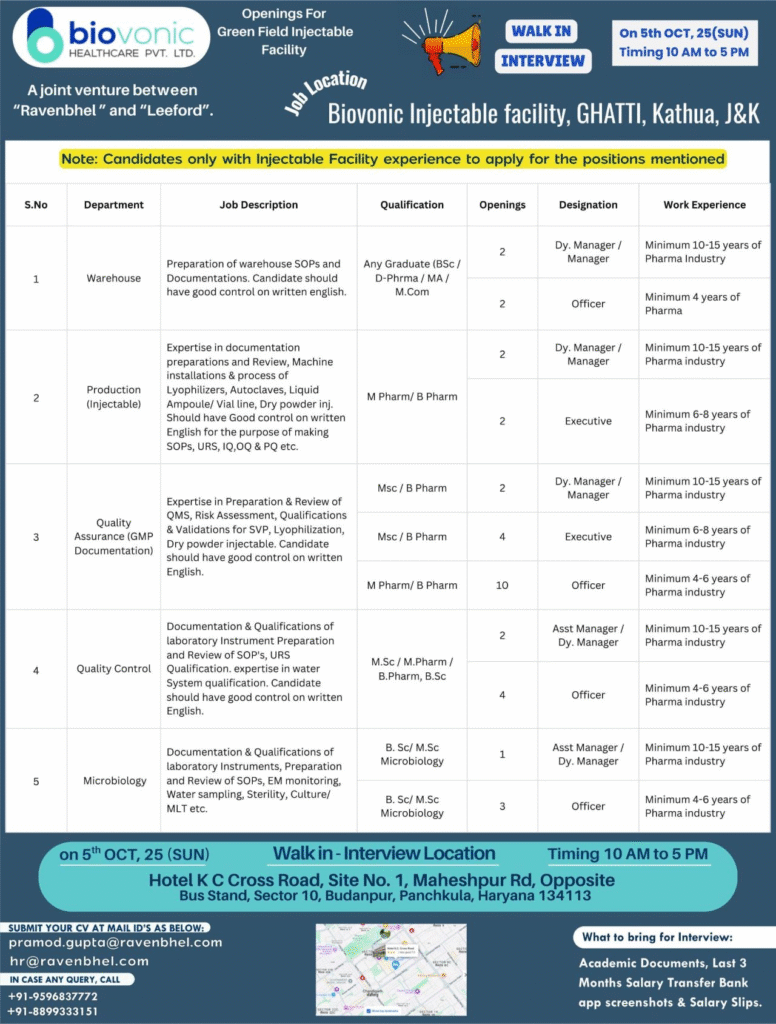
Walk-in Interview Details
- Date: October 5, 2025 (Sunday)
- Time: 10 AM to 5 PM
- Venue: Hotel K C Cross Road, Site No. 1, Maheshpur Rd, Opposite Bus Stand, Sector 10, Budanpur, Panchkula, Haryana 134113
- Contact/Email: pramod.gupta@ravenbhel.com, hr@ravenbhel.com; +91-9596837772, +91-8899333151
Why You Should Join
Biovonic Healthcare offers dynamic pharmaceutical careers in a green field injectable facility, backed by Ravenbhel and Leeford’s expertise. Gain hands-on experience in sterile manufacturing, lead compliance initiatives, and grow in an innovative, compliant culture.
With focus on quality and expansion in J&K, Biovonic ensures long-term stability and professional development. Discover more QA jobs, QC jobs, and production jobs at Ravenbhel Healthcare.
FAQs
Q: What experience is required for these pharma jobs at Biovonic?
A: Minimum 4-15 years in pharma industry, specifically injectable facilities; role-specific details apply.
Q: Where is the walk-in interview for pharmaceutical careers?
A: Hotel K C Cross Road, Panchkula, Haryana, on October 5, 2025, from 10 AM to 5 PM.
Q: What qualifications are needed for QA or QC roles?
A: B.Pharm, M.Pharm, B.Sc, or M.Sc; microbiology roles require B.Sc/M.Sc in Microbiology.
Q: What documents to bring for the interview?
A: Academic documents, last 3 months’ salary transfer bank app screenshots, and salary slips.
