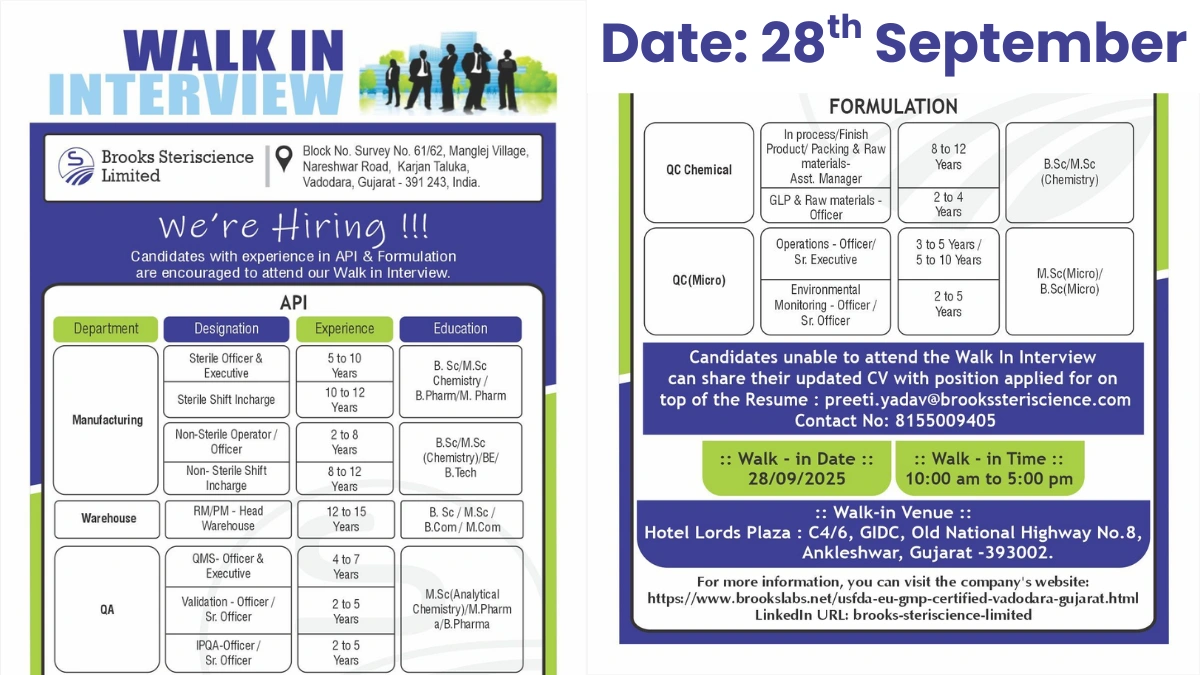
Brooks Steriscience Limited, a USFDA and EU GMP certified pharmaceutical leader, is hosting a walk-in interview for API and formulation roles. Join our state-of-the-art facility in Vadodara, Gujarat, specializing in sterile and non-sterile manufacturing. We drive innovation in global healthcare solutions.
Located in Karjan Taluka, our plant excels in high-quality APIs and formulations. With a focus on regulatory compliance, we serve international markets effectively. Explore our legacy at brookslabs.net.
Contents
About Brooks Steriscience Limited
Brooks Steriscience is renowned for sterile injectables and API production. Our Vadodara facility adheres to USFDA and EU GMP standards. We prioritize quality, safety, and sustainable practices. Our team supports global pharmaceutical demands.
We foster a collaborative culture with career growth opportunities. Connect with us on LinkedIn for updates. Join a trusted name in Gujarat’s pharma hub.
API Job Opportunities in Vadodara
Advance your career in API manufacturing with roles in sterile and non-sterile production. These positions require hands-on pharma experience. Work in a cutting-edge facility. Contribute to high-quality API output.
Manufacturing Positions
Manage sterile or non-sterile API processes. Oversee shift operations or execute production tasks. Ideal for chemistry and pharmacy professionals.
Key Requirements
- Sterile Officer & Executive
- Experience: 5-10 years
- Qualification: B.Sc/M.Sc Chemistry, B.Pharm/M.Pharm
- Skills: Sterile API manufacturing, process control
- Sterile Shift Incharge
- Experience: 10-12 years
- Qualification: B.Sc/M.Sc Chemistry, B.Pharm/M.Pharm
- Skills: Shift management, sterile operations
- Non-Sterile Operator/Officer
- Experience: 2-8 years
- Qualification: B.Sc/M.Sc Chemistry, BE/B.Tech
- Skills: Non-sterile API production
- Non-Sterile Shift Incharge
- Experience: 8-12 years
- Qualification: B.Sc/M.Sc Chemistry, BE/B.Tech
- Skills: Shift supervision, process optimization
- Location: Karjan, Vadodara, Gujarat
Warehouse Positions
Lead raw material and packaging material management. Ensure efficient inventory control. Suited for experienced logistics professionals.
Key Requirements
- Designation: RM/PM-Head Warehouse
- Experience: 12-15 years
- Qualification: B.Sc/M.Sc, B.Com/M.Com
- Skills: Warehouse management, supply chain
- Location: Karjan, Vadodara, Gujarat
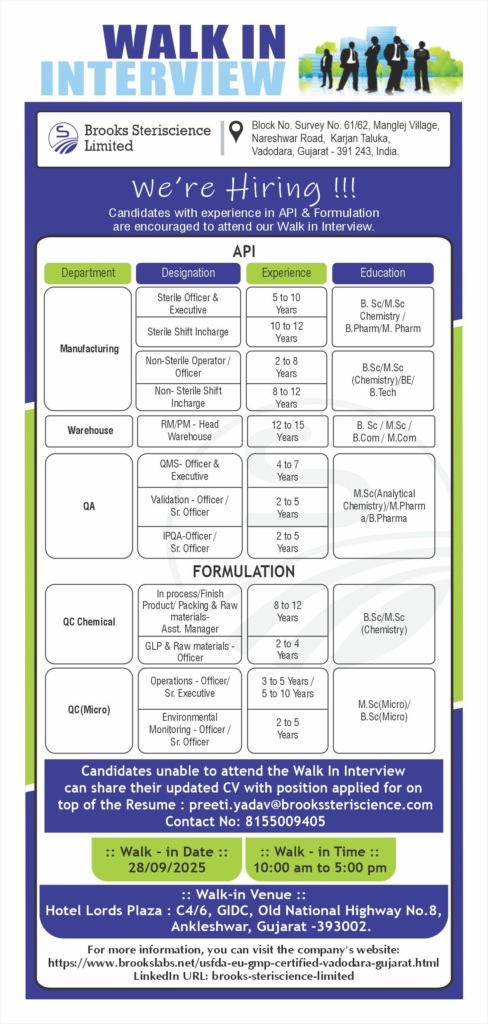
Formulation Job Opportunities
Support quality assurance and control in formulation processes. Focus on in-process checks, validation, and microbial testing. Ideal for detail-oriented professionals. Ensure compliance in injectable production.
Quality Assurance (QMS, Validation, IPQA)
Oversee QMS, equipment validation, and in-process quality. Support regulatory audits.
Key Requirements
- QMS-Officer & Executive
- Experience: 4-7 years
- Qualification: M.Sc (Analytical Chemistry), M.Pharm, B.Pharm
- Skills: QMS documentation, GMP compliance
- Validation-Officer/Sr. Officer
- Experience: 2-5 years
- Qualification: M.Sc (Analytical Chemistry), M.Pharm, B.Pharm
- Skills: Equipment/process validation
- IPQA-Officer/Sr. Officer
- Experience: 2-5 years
- Qualification: M.Sc (Analytical Chemistry), M.Pharm, B.Pharm
- Skills: In-process quality checks
- Location: Karjan, Vadodara, Gujarat
Quality Control (Chemical & Microbiology)
Conduct chemical and microbial testing for formulations. Manage GLP and environmental monitoring.
Key Requirements
- QC Chemical – Asst. Manager (In-process/Finished Product Packing & Raw Materials)
- Experience: 8-12 years
- Qualification: B.Sc/M.Sc Chemistry
- Skills: HPLC, GC, raw material testing
- QC Chemical – Officer (GLP & Raw Materials)
- Experience: 2-4 years
- Qualification: B.Sc/M.Sc Chemistry
- Skills: Good Laboratory Practices, testing
- QC Microbiology – Operations Officer/Sr. Executive
- Experience: 3-5 years / 5-10 years
- Qualification: B.Sc/M.Sc Microbiology
- Skills: Microbial testing, sterility assurance
- QC Microbiology – Environmental Monitoring Officer/Sr. Officer
- Experience: 2-5 years
- Qualification: B.Sc/M.Sc Microbiology
- Skills: Environmental monitoring, contamination control
- Location: Karjan, Vadodara, Gujarat
Walk-In Interview Details
Join our walk-in drive for API and formulation roles. Only experienced candidates are eligible. Bring your credentials for immediate consideration.
- Date: Sunday, September 28, 2025
- Time: 10:00 AM – 5:00 PM
- Venue: Hotel Lords Plaza, C4/6, GIDC, Old National Highway No.8, Ankleshwar, Gujarat – 393002
- What to Bring: Updated CV (mention position applied for), academic/experience documents
- Contact: Preeti Yadav at 8155009405 or preeti.yadav@brookssteriscience.com
Why Join Brooks Steriscience?
Brooks offers competitive pharma salaries in Vadodara, averaging INR 5-10 LPA. Work in a USFDA-certified facility with growth opportunities. Our culture supports professional development. Contribute to global healthcare solutions.
Application Tips for Pharma Careers
Research GMP standards for interviews. Highlight sterile API or formulation experience. Network on LinkedIn. Ensure CV specifies role applied for.
Join Vadodara’s thriving pharma ecosystem. For details, visit brookslabs.net.