The Central Drugs Standard Control Organisation (CDSCO) under the Ministry of Health & Family Welfare invites applications for the post of Assistant Drugs Controller (India) (Medical Devices). This is an excellent opportunity to contribute to India’s healthcare system in a regulatory role. Join us in ensuring the safety and efficacy of medical devices nationwide!
Contents
About CDSCO
CDSCO, part of the Ministry of Health & Family Welfare, regulates pharmaceuticals and medical devices in India. Our mission is to ensure quality, safety, and compliance with regulatory standards. The Assistant Drugs Controller role offers a chance to work in a dynamic, impactful environment.
Vacancy Details
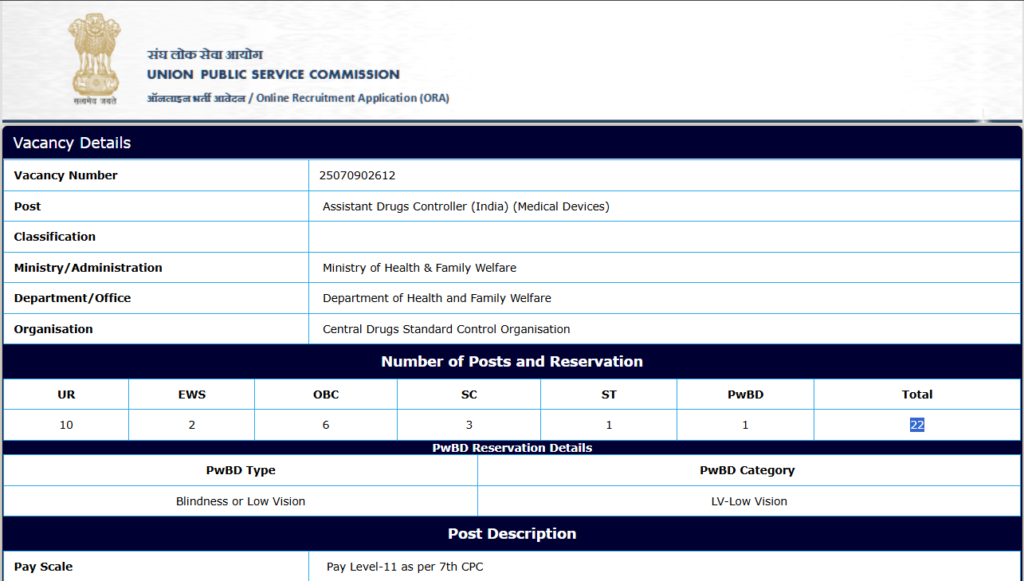
- Vacancy Number: 25070902612
- Post: Assistant Drugs Controller (India) (Medical Devices)
- Organization: Central Drugs Standard Control Organisation
- Location: FDA Bhawan, New Delhi (with potential postings across India)
- Post Type: General Central Service, Group A Gazetted (Non-Ministerial), Permanent
- Probation: 2 years with mandatory two-week induction training
Number of Posts and Reservation
| UR | EWS | OBC | SC | ST | PwBD | Total |
|---|---|---|---|---|---|---|
| 10 | 2 | 6 | 3 | 1 | 1 | 22 |
- PwBD Reservation: 1 post reserved for candidates with Blindness or Low Vision (LV-Low Vision)
Pay Scale and Age Limit
- Pay Scale: Pay Level-11 as per 7th CPC
Age Limit:
- Not exceeding 40 years for UR and EWS candidates
- Not exceeding 43 years for OBC candidates
- Not exceeding 45 years for SC/ST candidates
- Relaxable by up to 5 years for Central/U.T. Government servants
- Additional age concessions as per Government of India instructions
Essential Qualifications
Educational
- Option 1: Master’s degree in Bio-Medical Engineering, Chemical Engineering, Bio-Technology, Mechanical Engineering, Electrical Engineering, Electronics Engineering, Instrumentation Engineering, Polymer Engineering, Computer Science Engineering, Medical Electronics Engineering, Pharmacy, Pharmaceutical Sciences, Medicine with Clinical Pharmacology, Microbiology, Bio-Chemistry, Chemistry, or Life Sciences from a recognized University/Institute.
- Option 2: Bachelor’s degree in the above disciplines from a recognized University/Institute.
Experience
- For Master’s Degree Holders: 4 years of experience in manufacturing, testing, regulation, or designing of medical devices.
- For Bachelor’s Degree Holders: 6 years of experience in manufacturing, testing, regulation, or designing of medical devices.
Note: Qualifications and experience are relaxable at the discretion of the Union Public Service Commission (UPSC) for well-qualified candidates or SC/ST candidates if sufficient candidates are unavailable.
Duties and Responsibilities
- Coordinate and ensure compliance with directions from the Deputy Drugs Controller (India)
- Monitor Drugs Inspectors for joint inspections, complaint investigations, and sampling
- Process online applications and handle applicant queries
- Review, monitor, and evaluate files and inspection reports from Drugs Inspectors, Technical Officers, and Assistant Drugs Inspectors
- Participate in committees, workshops, and seminars as a technical expert or speaker
- Perform additional tasks assigned by the Drugs Controller General (India)
Why Join CDSCO?
CDSCO offers a prestigious platform to contribute to public health by regulating medical devices. Work in a regulatory-compliant environment with opportunities to engage in high-impact projects, audits, and technical discussions. The role provides career stability and growth within a vital government organization.
How to Apply
Interested candidates should apply through the UPSC portal. Ensure eligibility as per the stated qualifications and experience. For detailed application instructions, visit UPSC Online. For more about CDSCO, explore CDSCO Official Website.
Verified by Trusted HRs
The post is released by the UPSC Webpage. Click here to visit the post
How to Apply for Assistant Drugs Controller (India) at CDSCO
Follow these simple steps to apply for the Assistant Drugs Controller (India) (Medical Devices) position with the Central Drugs Standard Control Organisation (CDSCO):
- Check Eligibility: Ensure you meet the educational (Master’s/Bachelor’s in specified fields) and experience requirements (4 years for Master’s, 6 years for Bachelor’s in medical device manufacturing, testing, regulation, or design). Verify age limits and reservation criteria.
- Visit UPSC Portal: Go to the official UPSC application website at UPSC Online.
- Register/Login: Create an account on the UPSC portal or log in if you already have one. Complete the One-Time Registration (OTR) process if required.
- Find Vacancy: Locate the advertisement for Vacancy Number 25070902612 under the “Recruitment” section. Read the detailed notification carefully.
- Fill Application Form: Complete the online application form with accurate personal, educational, and professional details. Ensure all fields are correctly filled.
- Upload Documents: Upload scanned copies of required documents, including educational certificates, experience proofs, ID, and any category-specific certificates (e.g., EWS, OBC, SC/ST, PwBD).
- Pay Application Fee: Pay the applicable fee (if required) through the online payment gateway. Fee exemptions may apply for certain categories as per UPSC guidelines.
- Review and Submit: Double-check all entered information and uploaded documents. Submit the application before the closing date.
- Download Confirmation: Save and print the application confirmation for future reference.
- Monitor Updates: Regularly check the UPSC portal and your registered email for updates on the selection process or further instructions.
For detailed guidelines, visit UPSC Official Website or CDSCO Official Website. Apply promptly to join CDSCO’s mission of ensuring medical device safety!
Be a Part of Public Health Excellence
Seize this opportunity to join CDSCO as an Assistant Drugs Controller (India). Contribute to ensuring the safety and quality of medical devices in India. Apply now and make a difference in healthcare regulation!


