Cipla, a global pharmaceutical leader with a legacy of over 85 years, is committed to “Caring for Life” through innovative and accessible healthcare solutions. We are excited to announce walk-in interviews for Operator Production – API and Officer Production – API roles at our state-of-the-art facility in Kurkumbh, Pune. Join our team and contribute to delivering high-quality Active Pharmaceutical Ingredients (APIs) to global markets.
Contents
Why Work at Cipla?
At Cipla, we foster a culture of excellence, collaboration, and purpose-driven innovation. Our WHO-GMP certified facilities and commitment to Environment, Health, and Safety (EHS) standards provide a dynamic environment for professional growth.
- Work with a globally recognized pharmaceutical leader operating in 80+ countries
- Contribute to a diverse portfolio of over 1,500 products across multiple therapeutic categories
- Gain hands-on experience in cGMP-compliant API manufacturing
- Be part of a team dedicated to making healthcare affordable and accessible
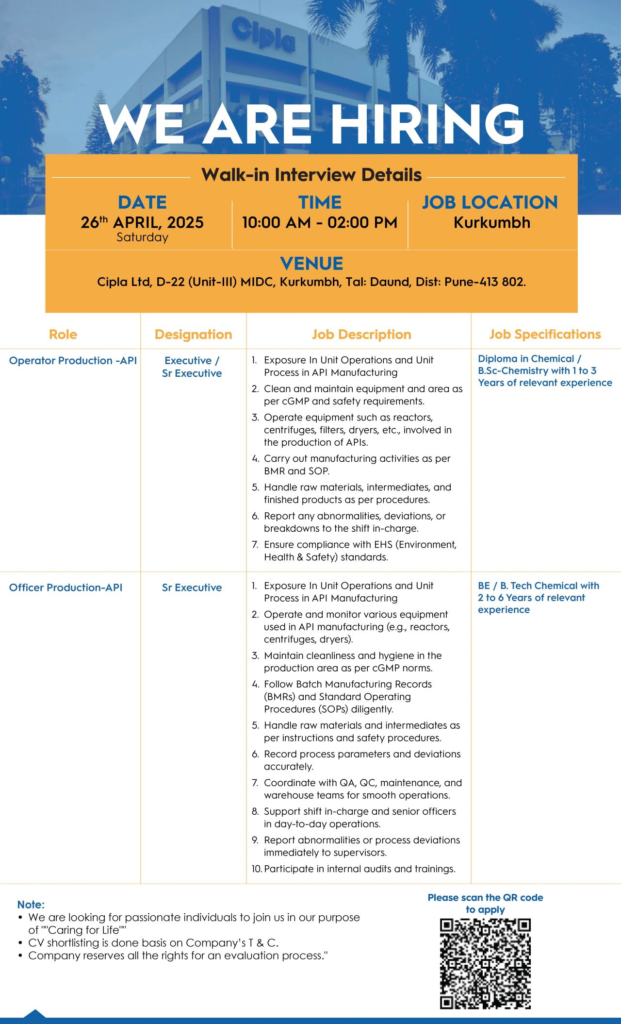
Walk-In Interview Details
- Date: April 26, 2025 (Saturday)
- Time: 10:00 AM – 2:00 PM
- Venue: Cipla Ltd., D-22 (Unit-III), MIDC, Kurkumbh, Tal: Daund, Dist: Pune, Maharashtra 413802
- Requirements: Bring an updated CV, passport-size photo, copies of educational certificates, Aadhar, PAN, latest CTC document, 3 months’ payslips, and 3 months’ bank statements
- Application: Scan the QR code provided in the original job posting to apply or attend the walk-in interview
Note: CV shortlisting will be based on Cipla’s terms and conditions. The company reserves all rights for the evaluation process. Cipla does not request any payment from candidates for employment.
Open Positions
We are hiring passionate individuals with relevant experience in API manufacturing for the following roles at our Kurkumbh facility. All positions require adherence to cGMP, EHS standards, and excellent communication skills.
1. Operator Production – API
- Designation: Executive / Senior Executive
- Qualification:
- Diploma in Chemical Engineering
- B.Sc. Chemistry
- Experience: 1-3 years in API manufacturing
Job Responsibilities:
- Operate equipment such as reactors, centrifuges, filters, and dryers involved in API production
- Perform unit operations and processes as per Batch Manufacturing Records (BMR) and Standard Operating Procedures (SOPs)
- Clean and maintain equipment and production areas per cGMP and safety requirements
- Handle raw materials, intermediates, and finished products following safety protocols
- Report abnormalities, deviations, or equipment breakdowns to the shift in-charge
- Ensure compliance with Environment, Health, and Safety (EHS) standards
2. Officer Production – API
- Designation: Senior Executive
- Qualification:
- B.E. / B.Tech in Chemical Engineering
- B.Sc. / M.Sc. Chemistry
- Experience: 2-6 years in API manufacturing
Job Responsibilities:
- Operate and monitor equipment (e.g., reactors, centrifuges, dryers) used in API manufacturing
- Maintain cleanliness and hygiene in the production area per cGMP norms
- Follow BMRs and SOPs diligently, ensuring accurate documentation of process parameters
- Handle raw materials and intermediates per safety and procedural guidelines
- Coordinate with QA, QC, maintenance, and warehouse teams for seamless operations
- Support shift in-charge and senior officers in daily production activities
- Report process deviations or abnormalities to supervisors immediately
- Participate in internal audits and training programs
How to Apply
Attend our walk-in interview on April 26, 2025, from 10:00 AM to 2:00 PM at our Kurkumbh facility. Alternatively, apply by scanning the QR code provided in the original job Caring for Life by joining Cipla’s production team in Kurkumbh. We look forward to welcoming passionate individuals to our mission of making healthcare accessible and affordable.
For more information about Cipla, visit www.cipla.com.



Intrested