Discover exciting pharma jobs at Cliantha Research in Ahmedabad. Join a leading CRO offering pharmaceutical careers in India with roles in clinical data, QA, medical writing, and more.
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
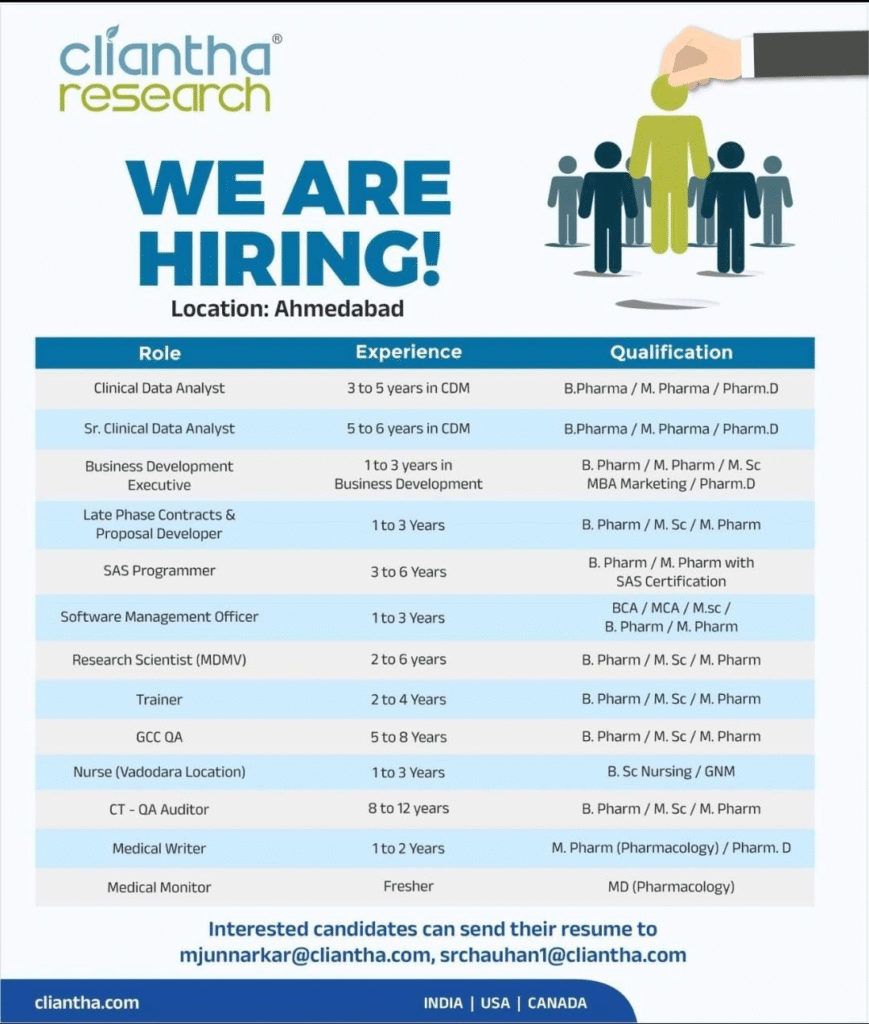
- 3.1 Clinical Data Analyst
- 3.2 Sr. Clinical Data Analyst
- 3.3 Business Development Executive
- 3.4 Late Phase Contracts & Proposal Developer
- 3.5 SAS Programmer
- 3.6 Software Management Officer
- 3.7 Research Scientist (MDMV)
- 3.8 Trainer
- 3.9 GCC QA
- 3.10 Nurse (Vadodara Location)
- 3.11 CT-QA Auditor
- 3.12 Medical Writer
- 3.13 Medical Monitor
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Why You Should Join
- 9 FAQs
About the Company
Cliantha Research is a full-service Clinical Research Organization (CRO) headquartered in Ahmedabad, India. With over 21 years of excellence, it maintains an impeccable regulatory history with global agencies including USFDA, WHO, MHRA, and Health Canada.
The company delivers end-to-end services in early-phase and late-phase clinical trials, bioanalytics, data management, biostatistics, and specialized areas like respiratory and dermatology research. Cliantha operates with the motto “Science with Integrity,” emphasizing innovation, regulatory compliance, and high-quality research standards.
It has a strong global presence with operations in India, USA, and Canada, making it a trusted partner in the pharmaceutical and biotech industries.
Job Details
- Company Name: Cliantha Research
- Experience: Varies by role (1–12 years)
- Qualification: B.Pharm, M.Pharm, Pharm.D, M.Sc, B.Sc Nursing, GNM, BCA/MCA, MD (Pharmacology), SAS Certification (where applicable)
- Location: Ahmedabad (primary); Vadodara (for Nurse role)
Job Description
Cliantha Research is actively hiring talented professionals to strengthen its clinical research and support functions. These pharmaceutical careers in India span data management, quality assurance, business development, programming, and medical services.
The openings provide opportunities to contribute to global clinical trials in a compliant and innovative environment.
Clinical Data Analyst
- Experience: 3 to 5 years in CDM
- Education/Qualification: B.Pharm / M.Pharm / Pharm.D
Sr. Clinical Data Analyst
- Experience: 5 to 6 years in CDM
- Education/Qualification: B.Pharm / M.Pharm / Pharm.D
Business Development Executive
- Experience: 1 to 3 years in Business Development
- Education/Qualification: B.Pharm / M.Pharm / M.Sc / MBA Marketing / Pharm.D
Late Phase Contracts & Proposal Developer
- Experience: 1 to 3 years
- Education/Qualification: B.Pharm / M.Sc / M.Pharm
SAS Programmer
- Experience: 3 to 6 years
- Education/Qualification: B.Pharm / M.Pharm with SAS Certification
Software Management Officer
- Experience: 1 to 3 years
- Education/Qualification: BCA / MCA / M.Sc / B.Pharm / M.Pharm
Research Scientist (MDMV)
- Experience: 2 to 6 years
- Education/Qualification: B.Pharm / M.Sc / M.Pharm
Trainer
- Experience: 2 to 4 years
- Education/Qualification: B.Pharm / M.Sc / M.Pharm
GCC QA
- Experience: 5 to 8 years
- Education/Qualification: B.Pharm / M.Sc / M.Pharm
Nurse (Vadodara Location)
- Experience: 1 to 3 years
- Education/Qualification: B.Sc Nursing / GNM
CT-QA Auditor
- Experience: 8 to 12 years
- Education/Qualification: B.Pharm / M.Sc / M.Pharm
Medical Writer
- Experience: 1 to 2 years
- Education/Qualification: M.Pharm (Pharmacology) / Pharm.D
Medical Monitor
- Experience: Fresher
- Education/Qualification: MD (Pharmacology)
Skills/Qualifications
- Strong educational background in pharmacy (B.Pharm, M.Pharm, Pharm.D) or life sciences (M.Sc)
- Relevant experience in clinical data management, quality assurance, or SAS programming
- SAS certification for programming roles
- Knowledge of regulatory guidelines (ICH-GCP, FDA, WHO)
- Excellent communication and analytical skills
- Proficiency in clinical trial processes and documentation
- Attention to detail and ability to work in a fast-paced CRO environment
Key Responsibilities
- Manage and review clinical trial data for accuracy
- Develop contracts and proposals for late-phase studies
- Perform quality audits and ensure GCP compliance
- Program and validate datasets using SAS
- Prepare medical and regulatory documents
- Conduct training sessions on clinical processes
- Monitor safety and medical aspects of trials
- Support business development initiatives
Benefits/Perks
- Competitive salary packages
- Opportunities for global exposure
- Continuous learning and professional development
- Supportive and collaborative work culture
- Career growth in a leading CRO
- Exposure to cutting-edge clinical research
How to Apply
Interested candidates should send their updated resume to mjunnarkar@cliantha.com or srchauhan1@cliantha.com. Mention the position applied for in the subject line. Visit the official website at cliantha.com for more details.
For additional pharma job opportunities, explore Pharma Recruiter. Don’t miss this chance to advance your pharmaceutical career in India – apply today!
Why You Should Join
Cliantha Research offers a dynamic environment where innovation meets regulatory excellence. Employees benefit from long-term career stability, ongoing training, and participation in global clinical trials.
The company’s commitment to integrity and compliance creates a professional culture focused on growth and recognition. Join a team that drives meaningful advancements in healthcare.
FAQs
What qualifications are required for these pharma jobs?
Most roles require B.Pharm, M.Pharm, Pharm.D, or M.Sc; specific certifications like SAS apply to certain positions.
Is there a walk-in interview for these openings?
No, applications are accepted via email only.
What is the application process?
Email your resume to the provided IDs with the role in the subject line.
Are there growth opportunities at Cliantha Research?
Yes, the company offers competitive salaries, global exposure, and strong career progression in clinical research.


