Explore multiple pharma jobs with walk-in interviews at Concord Biotech Limited. Opportunities in Production, Quality Control, Quality Assurance, Regulatory Affairs, Analytical Development, and Packaging for freshers and experienced professionals in Ahmedabad/Dholka.
Contents
About the Company
Concord Biotech Limited leads R&D-driven biopharma, specializing in fermentation-based Active Pharmaceutical Ingredients (APIs) and formulations. The company operates state-of-the-art facilities in Valthera/Dholka near Ahmedabad, Gujarat.
Multiple units hold approvals from USFDA, EU GMP, WHO, and other global regulators. Concord exports to over 70 countries, including regulated markets, with focus on innovation and quality compliance.
It provides dynamic pharmaceutical careers in India within a growth-oriented, compliant environment.
Job Details
- Company Name: Concord Biotech Limited
- Experience: Freshers to 12 years (varies by role)
- Qualification: B.Sc/M.Sc, B.Pharm/M.Pharm, ITI/Diploma, B.Com/M.Com (varies by role)
- Location: Valthera/Dholka, Ahmedabad District, Gujarat
- Work Type: On-site
Job Description
Concord Biotech conducts multiple walk-in drives in December 2025 for API, formulations, and injectables units. Roles span manufacturing, quality, regulatory, and analytical functions in GMP-compliant facilities.
Opportunities suit professionals seeking growth in biotechnology and sterile operations.
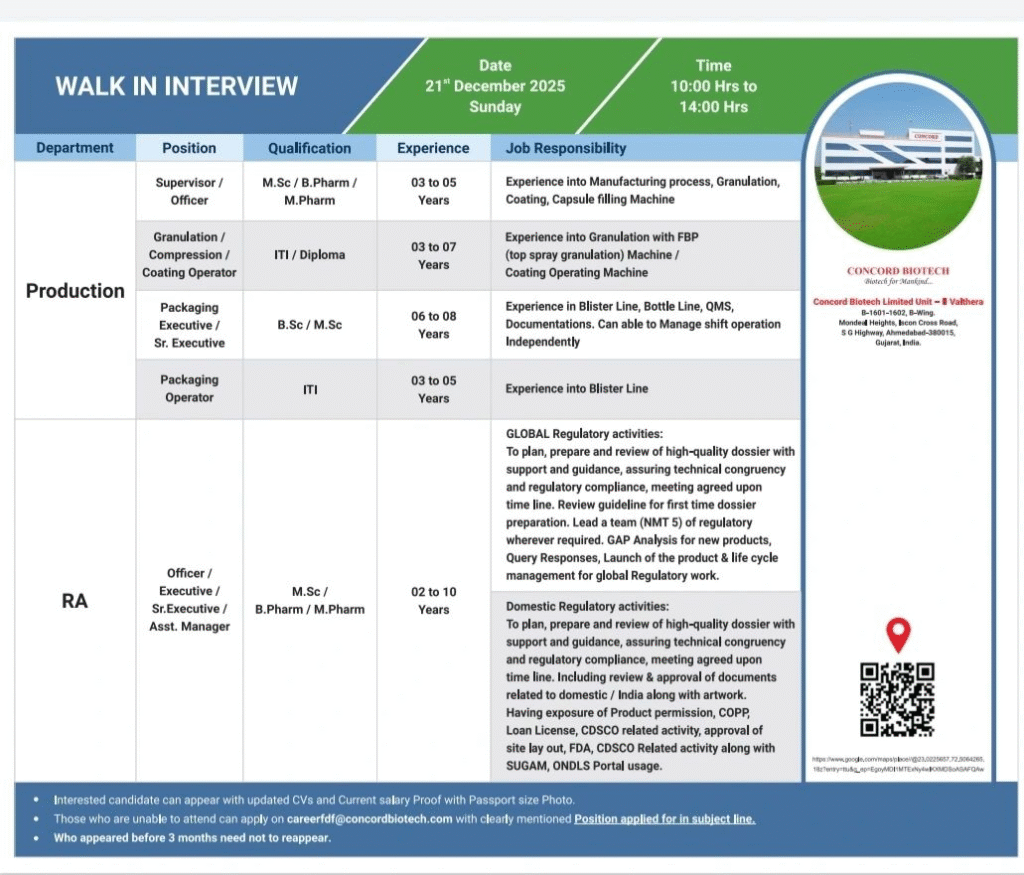
Production & Packaging Roles
- Officer/Supervisor Qualification: M.Sc/B.Pharm/M.Pharm Experience: 3–5 years Responsibilities: Manufacturing processes, granulation, coating, capsule filling
- Granulation/Compression/Coating Operator Qualification: ITI/Diploma Experience: 3–7 years Responsibilities: FBP top spray granulation, coating machines
- Packaging Executive/Sr. Executive Qualification: B.Sc/M.Sc Experience: 6–8 years Responsibilities: Blister/bottle lines, QMS, documentation, independent shift management
- Packaging Operator Qualification: ITI Experience: 3–5 years Responsibilities: Blister line operations
- Aseptic Production Roles (Operator/Officer/Executive/Sr. Executive) Qualification: ITI/B.Pharm/M.Pharm/B.Sc/M.Sc Experience: 2–10 years Responsibilities: Aseptic filling, vial washing, autoclave, lyophilizer, dry powder filling
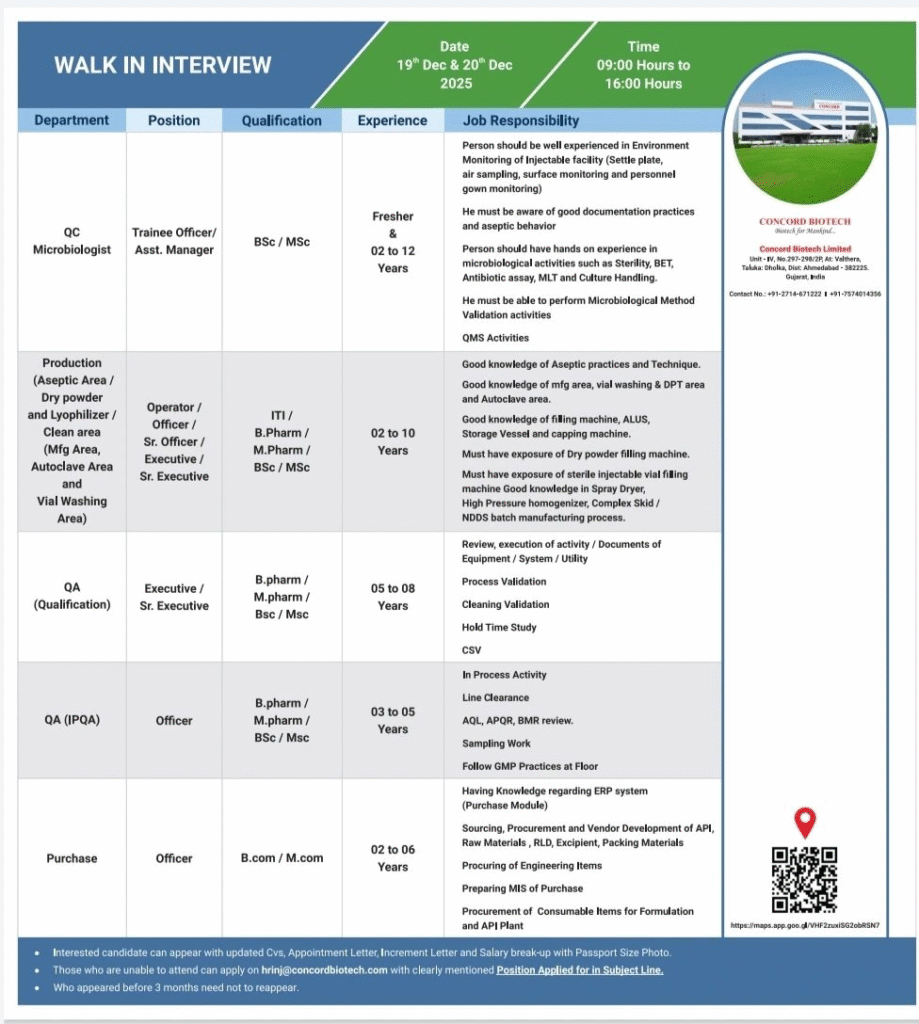
Quality Control & Microbiology
- QC Microbiologist (Trainee Officer/Asst. Manager) Qualification: B.Sc/M.Sc Experience: Freshers & 2–12 years Responsibilities: Environment monitoring, sterility, BET, MLT, culture handling, method validation
Quality Assurance
- QA Qualification (Executive/Sr. Executive/Officer) Qualification: B.Pharm/M.Pharm/B.Sc/M.Sc Experience: 3–8 years Responsibilities: Equipment/utility qualification, process/cleaning validation, CSV, hold time studies
- QA IPQA Roles Responsibilities: In-process checks, line clearance, AQL, sampling, GMP compliance
Regulatory Affairs
- Officer/Executive/Sr. Executive/Asst. Manager Qualification: M.Sc/B.Pharm/M.Pharm Experience: 2–10 years Responsibilities: Global/domestic dossier preparation, compliance, query responses, CDSCO activities, lifecycle management
Analytical Development Laboratory (ADL – Injectables)
- Officer/Sr. Officer Qualification: B.Sc/M.Sc/B.Pharm/M.Pharm Experience: 4–6 years
- Executive/Sr. Executive Qualification: B.Sc/M.Sc/B.Pharm/M.Pharm Experience: 6–8 years Responsibilities: Method development/validation/transfer, stability management, GLP/cGMP compliance, instrument handling (HPLC/GC/ICP)
Purchase
- Officer Qualification: B.Com/M.Com Experience: 2–6 years Responsibilities: Sourcing API/excipients/packing materials, vendor development, MIS, ERP purchase module
Skills/Qualifications
- Hands-on experience in granulation, coating, aseptic filling, or packaging lines
- Knowledge of GMP, aseptic practices, and regulatory guidelines
- Proficiency in microbiological testing, method validation, or analytical instruments
- Expertise in dossier compilation, QMS, validations, or procurement
- Strong documentation, investigation, and teamwork skills
- Familiarity with CDSCO, USFDA requirements, and portals like SUGAM
Key Responsibilities
- Execute manufacturing and packaging operations
- Perform aseptic filling and environment monitoring
- Conduct microbiological and analytical testing
- Handle qualifications, validations, and IPQA
- Prepare regulatory dossiers and responses
- Manage stability studies and method development
- Ensure GMP compliance and documentation
- Support procurement and vendor management
Benefits/Perks
- Work in USFDA/EU GMP-approved facilities
- Exposure to fermentation, injectables, and global markets
- Professional growth and training programs
- Collaborative innovation-focused culture
- Career progression in R&D-driven organization
- Learning opportunities in regulatory compliance
- Long-term stability with global leader
How to Apply
Attend the respective walk-in with updated CV, salary proof, increment/appointment letter, and passport photo. Alternatively, email specified address with position in subject line.

Candidates interviewed in last 3 months should not reappear. Advance your biotech/pharma career—apply now! For more pharma jobs, visit Pharma Recruiter.
Walk-in Interview Details
Walk-in for Production, Packaging & RA (Formulations)
- Date: 21 December 2025 (Sunday)
- Time: 10:00 AM to 02:00 PM
- Venue: B-1601-1602, B-Wing, Monded Heights, SG Highway, Ahmedabad – 380015, Gujarat
- Email:careerfdf@concordbiotech.com
Walk-in for QC Microbiology, Aseptic Production, QA & Purchase (Injectables)
- Dates: 19th & 20th December 2025
- Time: 09:00 AM to 04:00 PM
- Venue: Concord Biotech Limited Unit-IV, No. 297-298/2, At: Valthera, Tal: Dholka, Dist: Ahmedabad – 382225, Gujarat
- Contact: +91-2714-671222 / +91-7574014358
- Email:hrinj@concordbiotech.com
Walk-in for ADL Injectable Analyst
- Dates: 19th & 20th December 2025
- Time: 09:00 AM to 04:00 PM
- Venue: Concord Biotech Limited Unit-V, No. 297-298/27, At: Valthera, Tal: Dholka, Dist: Ahmedabad – 382225, Gujarat
- Contact: +91-2714-671222 / +91-7574014356
- Email:hrinj@concordbiotech.com
Why You Should Join
Concord Biotech offers rewarding careers in a leading fermentation-based API company. Employees gain experience in USFDA-approved facilities and global regulatory environments.
The organization promotes innovation, quality excellence, and professional development. Join a trusted name delivering high-quality pharmaceuticals worldwide. Build stable, long-term growth in Gujarat’s thriving pharma hub.
FAQs
What experience is required?
Roles range from freshers (QC Micro) to 12 years; specific requirements vary by position.
Can I apply via email if unable to attend?
Yes, send CV to the respective email (careerfdf@ or hrinj@concordbiotech.com) mentioning position.
Who should not attend?
Candidates who appeared for interview in the last 3 months.
What growth opportunities exist?
Exposure to advanced biotech processes, regulatory submissions, and career progression in a globally compliant organization.


