Join Dishman Carbogen Amcis Ltd walk-in interview on 23rd November 2025 in Ahmedabad. Multiple openings in QA, QC, Instrumentation, Microbiology & Warehouse for API & Formulation – excellent pharma jobs in Gujarat!
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Quality Assurance (API & Formulation) – Officer to Assistant Manager
- 3.2 Quality Control (API & Formulation) – Sr. Officer to Sr. Executive
- 3.3 Quality Control – AMV (Executive to Deputy Manager)
- 3.4 Quality Control – Microbiology (Senior Officer/Executive)
- 3.5 Instrumentation & Control (Executive to Assistant Manager)
- 3.6 Instrumentation & Control (Technician)
- 3.7 Store (Officer to Deputy Manager)
- 3.8 Dispensing / Dispatch (Operator to Officer)
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
Dishman Carbogen Amcis Limited (DCAL) is a globally respected, fully-integrated CRAMS player with strong capabilities in Active Pharmaceutical Ingredients (API) and Formulations. Headquartered in Ahmedabad, the company operates USFDA, EU-GMP, and other international regulatory-approved facilities.
Known for innovation, high-quality standards, and end-to-end contract development & manufacturing services, Dishman offers rewarding pharmaceutical careers in India with global exposure and long-term growth opportunities.
Job Details
- Company Name: Dishman Carbogen Amcis Limited – Bavla Plant
- Experience: 2–12 years (varies by role)
- Qualification: B.Sc/M.Sc Chemistry, B.Pharm/M.Pharm, M.Sc Microbiology, BE/B.Tech (Mech/Elec/Inst), ITI, Graduate
- Location: Bavla, Ahmedabad District, Gujarat
- Work Type: On-site
Job Description
Dishman Carbogen Amcis is conducting a mega walk-in drive for its state-of-the-art Bavla manufacturing plant. Multiple positions are open across Quality Assurance, Quality Control (including AMV & Microbiology), Instrumentation & Engineering, and Stores & Dispensing departments in API and Formulation segments.
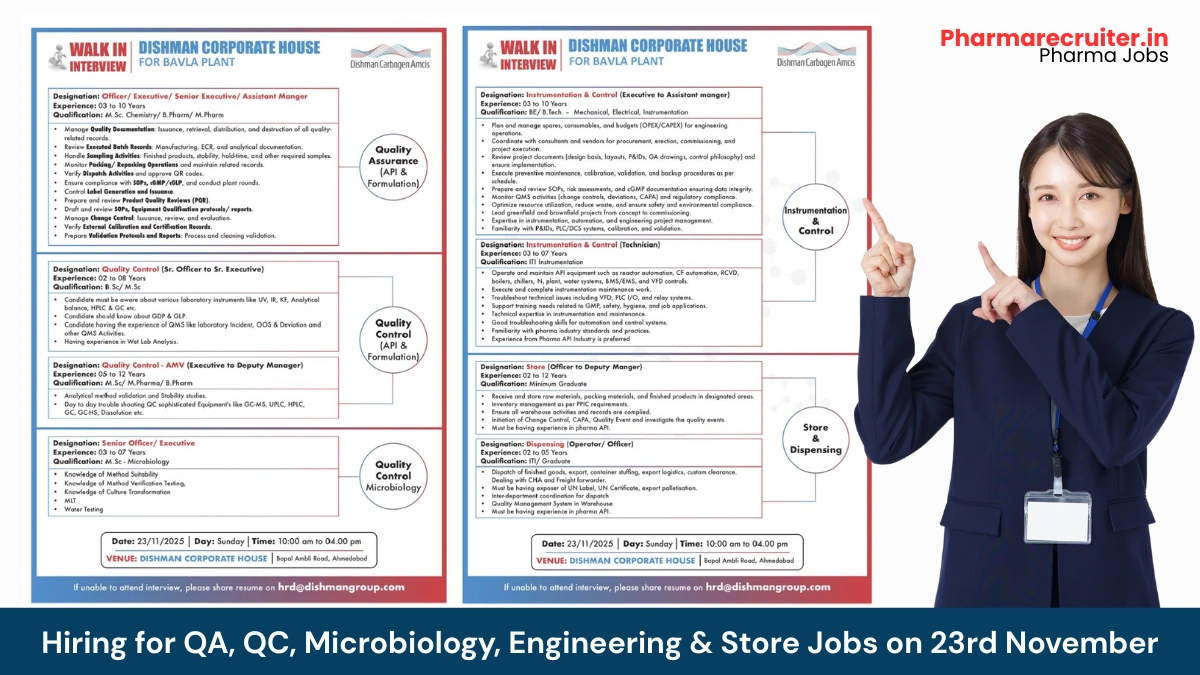
Quality Assurance (API & Formulation) – Officer to Assistant Manager
- Department: Quality Assurance
- Role: IPQA, Documentation & Compliance
- Experience: 3–10 years
- Education/Qualification: M.Sc Chemistry / B.Pharm / M.Pharm
Quality Control (API & Formulation) – Sr. Officer to Sr. Executive
- Department: Quality Control – Wet Lab & Instrumentation
- Role: Routine analysis & QMS handling
- Experience: 2–8 years
- Education/Qualification: B.Sc / M.Sc Chemistry
Quality Control – AMV (Executive to Deputy Manager)
- Department: Quality Control – Analytical Method Validation
- Role: Method validation, verification & stability studies
- Experience: 5–12 years
- Education/Qualification: M.Sc / M.Pharm / B.Pharm
Quality Control – Microbiology (Senior Officer/Executive)
- Department: QC Microbiology
- Role: Microbial Limit Testing, water analysis & culture handling
- Experience: 3–7 years
- Education/Qualification: M M.Sc Microbiology
Instrumentation & Control (Executive to Assistant Manager)
- Department: Engineering – Instrumentation
- Role: Project execution, maintenance & automation
- Experience: 3–10 years
- Education/Qualification: BE/B.Tech Mechanical / Electrical / Instrumentation
Instrumentation & Control (Technician)
- Department: Engineering – Instrumentation
- Role: Hands-on maintenance & troubleshooting
- Experience: 3–7 years
- Education/Qualification: ITI Instrumentation
Store (Officer to Deputy Manager)
- Department: Warehouse & Stores (API)
- Role: Inventory, receipt, dispensing & QMS
- Experience: 2–12 years
- Education/Qualification: Minimum Graduate
Dispensing / Dispatch (Operator to Officer)
- Department: Warehouse – Dispensing & Export Logistics
- Role: Raw material dispensing & finished goods dispatch
- Experience: 2–5 years
- Education/Qualification: ITI / Graduate
Skills/Qualifications
- Hands-on experience on HPLC, GC, GC-HS, UPLC, GC-MS, UV, IR, KF, Dissolution
- Analytical Method Validation, Method Verification & Stability studies
- QMS elements: Deviation, OOS, Laboratory Incident, Change Control, CAPA
- GMP, GLP, GDP, Data Integrity, cGMP documentation
- Instrumentation, PLC/DCS, VFD, P&ID, automation & calibration
- Microbiology: MLT, BET, water testing, culture handling
- Warehouse operations, export documentation, UN labeling, CHA coordination
- SOP drafting, risk assessment, validation protocols
Key Responsibilities
- Review batch records and quality documentation
- Perform IPQA and sampling activities
- Handle QMS elements and investigations
- Execute analytical testing and method validation
- Maintain and troubleshoot sophisticated instruments
- Conduct microbial limit tests and environmental monitoring
- Manage preventive maintenance and calibrations
- Oversee warehouse receipt, dispensing and dispatch
- Lead engineering projects and ensure regulatory compliance
- Prepare PQR, validation reports and SOPs
Benefits/Perks
- Competitive salary and performance incentives
- Excellent career growth in a global CRAMS organization
- Exposure to USFDA & EU-GMP approved plants
- Continuous learning and skill development programs
- Job stability with a reputed pharma group
- Dynamic and supportive work culture
How to Apply
Send your updated resume to hrd@dishmangroup.com if you cannot attend. Explore more pharma job opportunities on Pharma Recruiter. Apply today and take the next step in your pharmaceutical career!
Verified Post
The post is released by the Dishman Corporate House LinkedIn page. Click here to visit the post


Walk-in Interview Details
- Date: 23 November 2025 (Sunday)
- Time: 10:00 am to 04:00 pm
- Venue: Dishman Corporate House, Bopal-Ambli Road, Ahmedabad, Gujarat
- Email: hrd@dishmangroup.com
Why You Should Join
Dishman Carbogen Amcis is a trusted name in contract research and manufacturing with a strong track record of regulatory excellence and innovation. Employees enjoy global exposure, cutting-edge technology, structured growth paths, and a culture that values quality and compliance. Join a company that invests in its people and offers long-term stability in the fast-growing pharma sector.
FAQs
What positions are available in this Dishman walk-in interview?
Openings in QA, QC (including AMV & Microbiology), Instrumentation, Engineering, Stores & Dispensing for Bavla plant.
Is prior pharma API experience mandatory?
Yes, experience from Pharma API/FORMULATION industry is preferred for most roles.
Can freshers attend the Dishman walk-in on 23rd Nov 2025?
No, all positions require minimum 2–3 years of relevant experience.
What is the salary package and growth scope?
Highly competitive salary with excellent growth opportunities in a globally approved organization.