Dishman Carbogen Amcis Ltd, a renowned name in pharmaceutical contract manufacturing, invites talented professionals to attend walk-in interviews for multiple positions across Quality Control, Engineering, Production, Safety, and Stores departments. This is an excellent opportunity to advance your career in pharmaceutical manufacturing, API production, and pharma engineering.
Contents
Walk-In Interview Details
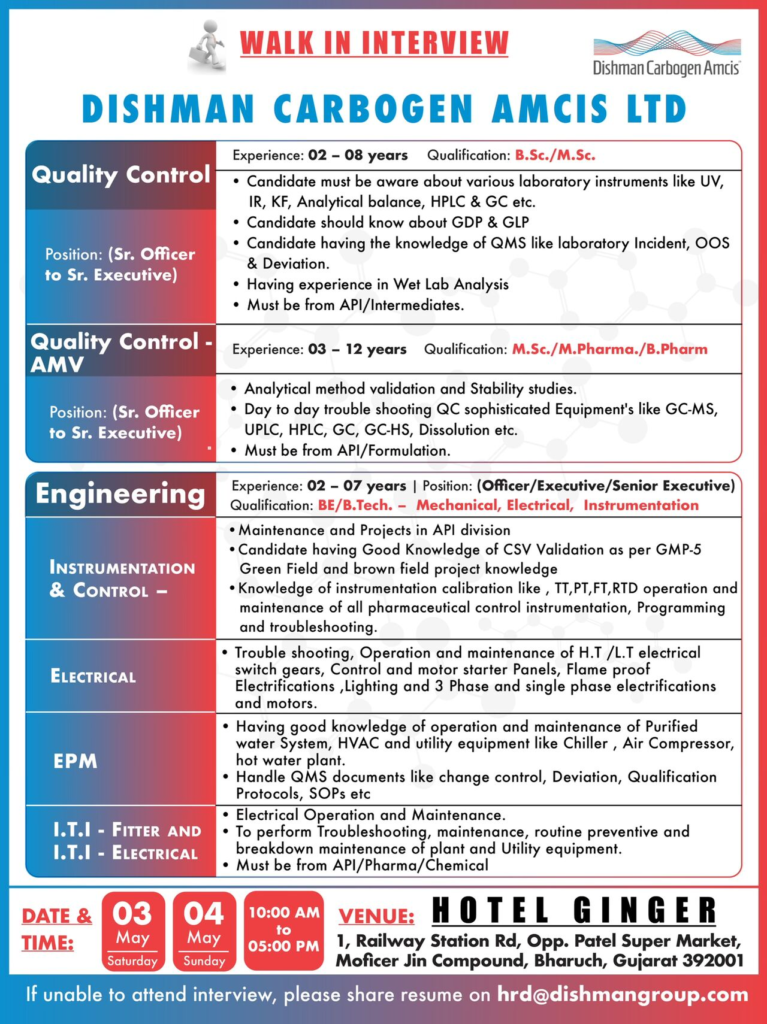
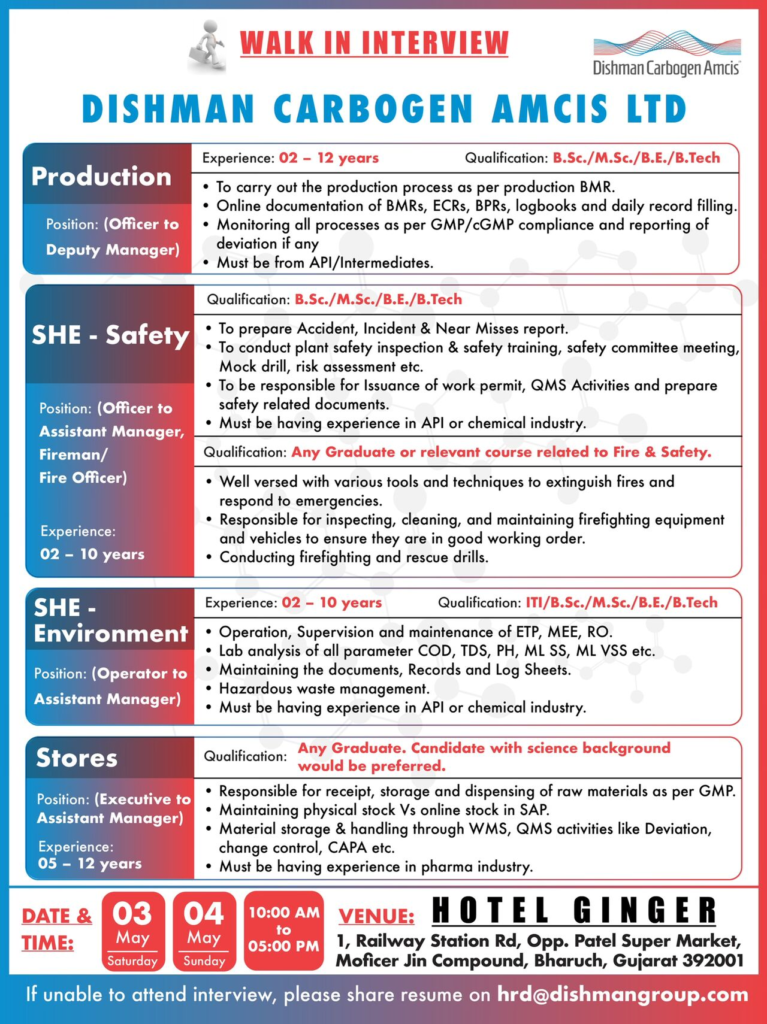
- Dates: 03 May 2025 (Saturday) & 04 May 2025 (Sunday)
- Time: 10:00 AM to 5:00 PM
- Venue: Hotel Ginger, 1 Railway Station Road, Opp. Patel Super Market, Moficer Jin Compound, Bharuch, Gujarat 392001
Available Positions and Candidate Requirements
Quality Control (QC) – Sr. Officer to Sr. Executive
- Experience: 2 to 12 years
- Qualification: B.Sc., M.Sc., B.Pharm, or M.Pharma
Key Skills:
- Proficient with laboratory instruments such as UV, IR, Karl Fischer, Analytical Balance, HPLC, GC, GC-MS, UPLC, GC-HS, and Dissolution apparatus
- Knowledge of Good Documentation Practices (GDP) and Good Laboratory Practices (GLP)
- Experience in wet lab analysis and managing Quality Management System (QMS) activities like laboratory incidents, Out of Specification (OOS), and deviations
- Expertise in analytical method validation and stability studies
- Experience in both API and formulation environments preferred
Engineering – Officer/Executive/Senior Executive
- Experience: 2 to 7 years
- Qualification: BE/B.Tech in Mechanical, Electrical, or Instrumentation Engineering
Key Skills:
- Maintenance and project execution in API manufacturing plants
- Good knowledge of Computer System Validation (CSV) as per GMP-5, including greenfield and brownfield projects
- Instrumentation calibration and troubleshooting (temperature transmitters, pressure transmitters, flow transmitters, RTD sensors)
- Operation and maintenance of HT/LT electrical switchgear, motor starter panels, flameproof electrification, lighting, and motors
- Maintenance of purified water systems, HVAC, chillers, air compressors, and hot water plants
- Handling QMS documentation such as change control, deviation reports, qualification protocols, and SOPs
- Experience in pharmaceutical, API, or chemical industries is essential
Production – Officer to Deputy Manager
- Experience: 2 to 12 years
- Qualification: B.Sc., M.Sc., B.E., or B.Tech
Key Responsibilities:
- Execute production processes as per Batch Manufacturing Records (BMR)
- Maintain online documentation of BMRs, Engineering Change Requests (ECRs), Batch Packing Records (BPRs), logbooks, and daily records
- Monitor all processes ensuring GMP/cGMP compliance and report deviations promptly
- Must have experience in API or intermediates manufacturing
Safety, Health & Environment (SHE)
SHE Safety Officer to Assistant Manager, Fireman/Fire Officer
- Experience: 2 to 10 years
- Qualification: B.Sc., M.Sc., B.E., B.Tech, or relevant Fire & Safety course
- Responsibilities include accident and incident reporting, conducting plant safety inspections, safety training, mock drills, risk assessments, work permit issuance, and QMS activities related to safety
- Must be experienced in API or chemical industries
- Firefighting skills and equipment maintenance are essential
SHE Environment Operator to Assistant Manager
- Experience: 2 to 10 years
- Qualification: ITI, B.Sc., M.Sc., B.E., or B.Tech
- Responsible for operation, supervision, and maintenance of Effluent Treatment Plant (ETP), Multiple Effect Evaporator (MEE), and Reverse Osmosis (RO) systems
- Conduct lab analysis of parameters such as COD, TDS, pH, MLSS, and VSS
- Maintain documentation, hazardous waste management, and compliance with environmental regulations
Stores – Executive to Assistant Manager
- Experience: 5 to 12 years
- Qualification: Any Graduate (Science background preferred)
Responsibilities:
- Manage receipt, storage, and dispensing of raw materials as per GMP
- Maintain physical and online stock records in SAP
- Oversee material storage and handling through Warehouse Management System (WMS)
- Handle QMS activities such as deviation, change control, and Corrective and Preventive Actions (CAPA)
- Experience in pharmaceutical industry preferred
Why Choose Dishman Carbogen Amcis?
- Work with a global pharmaceutical manufacturing leader specializing in API and formulation
- Gain hands-on experience with advanced QC instruments and pharma engineering systems
- Develop expertise in GMP/cGMP compliance, QMS documentation, and pharma safety protocols
- Opportunities for career growth in pharmaceutical production, quality control, engineering, and environmental health & safety
How to Apply
- Walk-In: Attend the interview on 3rd or 4th May with updated resume, educational certificates, and experience proof
- Unable to attend? Send your resume to hrd@dishmangroup.com
Important Notice: Dishman Carbogen Amcis Ltd does not charge any recruitment fees. Beware of fraudulent agents or offers requesting payment.
Take this opportunity to join Dishman Carbogen Amcis Ltd and build a rewarding career in the pharmaceutical industry!
